乳腺癌
乳腺癌是一種起源于乳腺上皮細胞的惡性腫瘤,是女性中最常見的癌癥之一,也是導致女性癌癥死亡的主要原因之一。乳腺癌的發病率與多種因素有關,包括遺傳、年齡、激素水平、生活方式等。根據腫瘤細胞的特征,乳腺癌可以分為不同的亞型,如激素受體陽性(ER+/PR+)、人表皮生長因子受體2(HER2+)和三陰性乳腺癌(TNBC)等。
近年來,乳腺癌的治療和藥物研發取得了顯著進展。特別是在靶向治療和免疫治療領域,新藥物和新療法的不斷涌現為患者帶來了更多的治療選擇和更好的預后。
靶向治療藥物
● HER2陽性乳腺癌
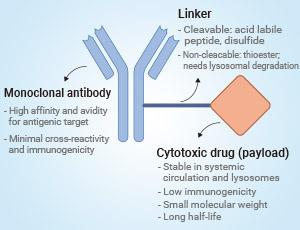
HER2陽性乳腺癌的治療在過去幾年中取得了重大進展。ADC(抗體藥物偶聯物)類藥物治療是HER2陽性乳腺癌治療的重要突破。例如,DS-8201(Trastuzumab Deruxtecan)是一種針對HER2的ADC藥物,已經在臨床試驗中顯示出對HER2低表達和HER2陰性乳腺癌患者的潛在療效67。此外,T-DM1(Trastuzumab Emtansine)和T-DXd(Trastuzumab Deruxtecan)也在HER2陽性乳腺癌的治療中取得了成功。
● 激素受體陽性乳腺癌
對于激素受體陽性的乳腺癌,CDK4/6抑制劑如阿貝西利(Abemaciclib)、達爾西利(Dalpiciclib)和瑞波西利(Ribociclib)等藥物的開發為患者提供了新的治療選擇。這些藥物通過抑制細胞周期的關鍵調節因子,減緩腫瘤細胞的生長和擴散。
● 三陰性乳腺癌
三陰性乳腺癌(TNBC)是一種較為難治的亞型,因為它缺乏激素受體和HER2的表達。然而,免疫治療在這一領域取得了進展,例如PD-1/PD-L1抑制劑在TNBC的治療中顯示出潛力。
免疫治療藥物
免疫治療是乳腺癌治療領域的另一個重要進展。免疫檢查點抑制劑,如PD-1和PD-L1抑制劑,已經在三陰性乳腺癌的治療中顯示出一定的療效。此外,研究者正在探索將免疫治療與其他治療方法結合使用的策略,以提高治療效果。
乳腺癌藥物靶點
- CA9
- CD247
- CD27
- CD274
- CD276
- CD28
- CD40
- CD44
- CD47
- CEACAM5
- CSF1R
- CSF2
- CSF3R
- CTAG1A
- CTLA4
- DNA2
- EGFR
- EpCAM
- EpoR
- ERBB2
- ERBB3
- FCGR1A
- FCY1
- FGFR3
- FOLR1
- HMMR
- ICAM1
- ICOS
- IGF1
- IGF1R
- IGF2
- IL12A
- IL12RB1
- IL15RA
- IL2
- IL2RA
- IL2RB
- KDR
- LAG3
- MELTF
- MET
- MSLN
- MUC1
- MUC16
- NECTIN4
- NMT1
- NMT2
- NT5E
- PDCD1
- PRLR
- PTK7
- PVRIG
- ROR1
- SIRPA
- STING1
- TACSTD2
- TF
- TGFB1
- TGFB2
- TIGIT
- TLR8
- TNFRSF10B
- TNFRSF1A
- TNFRSF4
- TNFSF11
- TNFSF9
- TOP1
- TPBG
- VEGFA
- VSIR
- VTCN1
乳腺癌藥物靶點相關產品推薦
● 靶點蛋白
CSB-MP007763HU
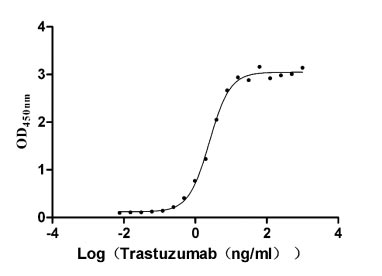
Measured by its binding ability in a functional ELISA. Immobilized HER2 at 2 μg/ml can bind Trastuzumab, the EC50 is 2.179-2.825 ng/ml.
CSB-MP619964HU1
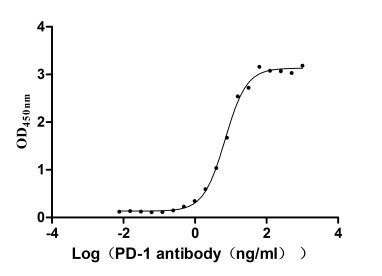
Measured by its binding ability in a functional ELISA. Immobilized PD-1 at 2 μg/ml can bind Anti-PD-1 recombinant antibody, the EC50 of human PD-1 protein is 6.087-7.854 ng/ml.
CSB-MP878942HU1
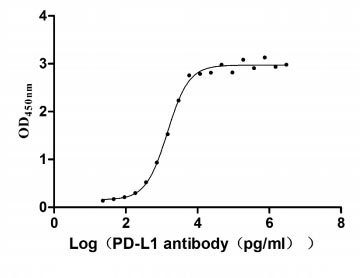
Measured by its binding ability in a functional ELISA. Immobilized PD-L1 at 2 μg/ml can bind Anti- PD-L1 mouse monoclonal antibody (CSB-MA878942A1m, antigen from E.coli), the EC50 of human PD-L1 protein is 1.252-1.653 ng/mL
CSB-MP023072HU1
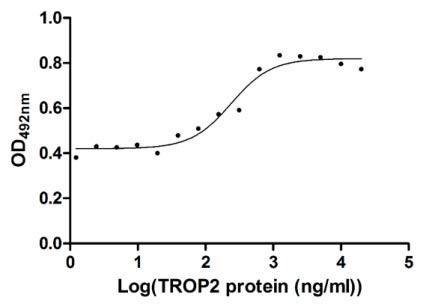
Measured in cell activity assay using U937 cells, the EC50 for this effect is 190.2-298.6 ng/ml.
CSB-MP007765HU
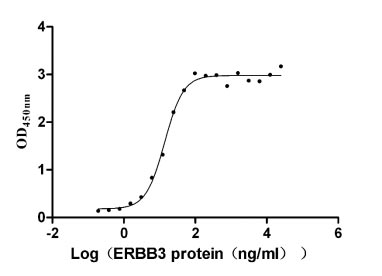
Measured by its binding ability in a functional ELISA. Immobilized NRG1 (CSB-MP016077HU1(F6)) at 2 μg/ml can bind human ERBB3, the EC50 is 12.32-15.74 ng/ml.
CSB-MP006163HU1
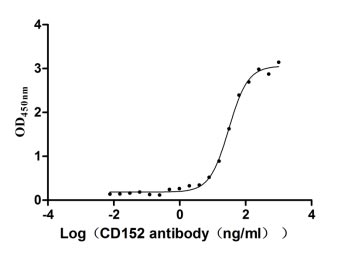
Measured by its binding ability in a functional ELISA. Immobilized CD152 at 2 μg/ml can bind Anti-CD152 rabbit monoclonal antibody (CSB-RA213310A0HU), the EC50 of human CD152 protein is 27.14-34.82 ng/ml.
CSB-MP004940HU
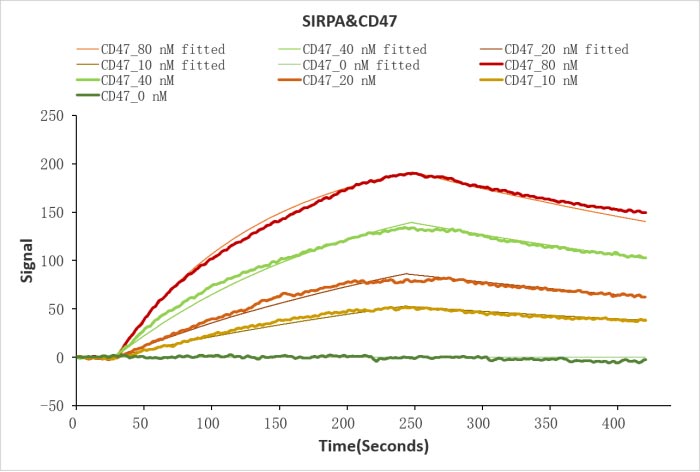
Human SIRPA protein His/Myc tag (CSB-MP021334HU) captured on COOH chip can bind Human CD47 protein Fc tag (CSB-MP004940HU) with an affinity constant of 19.1 nM as detected by LSPR Assay.
CSB-MP007479HU
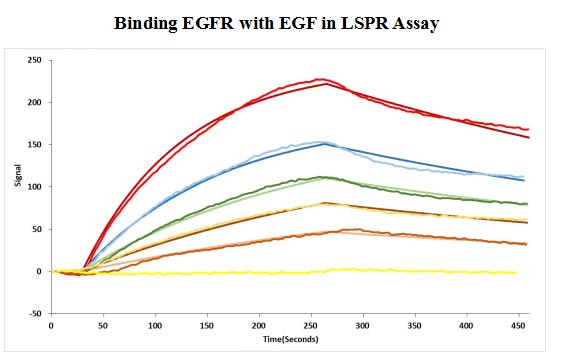
Human EGF protein captured on COOH chip can bind Human EGFR protein, his and Myc tag (CSB-MP007479HU) with an affinity constant of 11.9nM as detected by LSPR Assay.
● 穩定細胞株
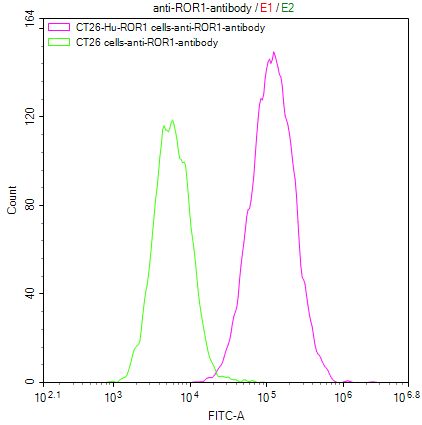
CT26/Human ROR1 Stable Cell Line
CSB-SC020067HU
Untransfected CT26 cells (green line) and transfected Human ROR1 CT26 stable cells (red line) were stained with anti-ROR1 antibody (CSB-RA020067A1HU) (2μg/1*106 cells), washed and then followed by FITC-conjugated anti-Human IgG Fc antibody and analyzed with flow cytometry.
● 重組抗體
CSB-RA260392A0HU
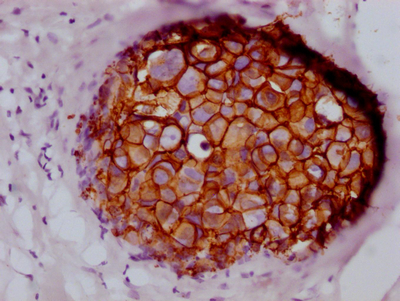
IHC image of CSB-RA260392A0HU diluted at 1:100 and staining in paraffin-embedded human breast cancer performed on a Leica BondTM system.
CSB-RA634199A0HU
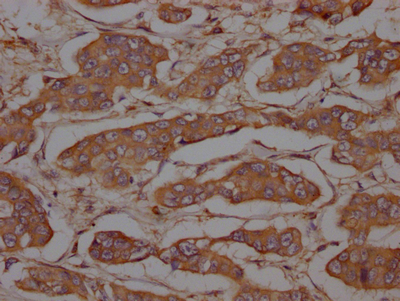
IHC image of CSB-RA634199A0HU diluted at 1:100 and staining in paraffin-embedded human breast cancer performed on a Leica BondTM system.
CSB-RA292372A0HU
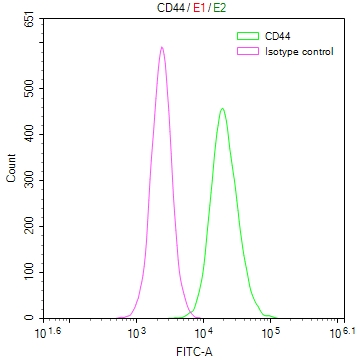
Overlay Peak curve showing Hela cells surface stained with CSB-RA292372A0HU (red line) at 1:50.
CSB-RA159341A0HU
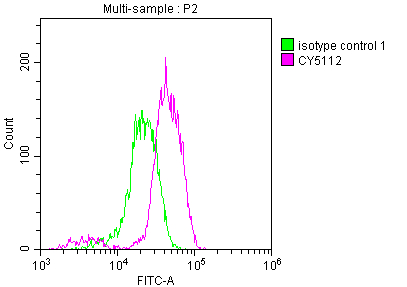
Overlay histogram showing Jurkat cells stained with CSB-RA159341A0HU (red line) at 1:50.
| 產品名稱 | 貨號 | 靶點 | 反應種屬 | 應用范圍 |
|---|---|---|---|---|
| CA9 Recombinant Monoclonal Antibody | CSB-RA614990A0HU | CA9 | Human | ELISA, IHC |
| CD247 Recombinant Monoclonal Antibody | CSB-RA244537A0HU | CD247 | Human | ELISA, FC |
| CD27 Recombinant Monoclonal Antibody | CSB-RA953976A0HU | CD27 | Human | ELISA, WB |
| CD274 Recombinant Monoclonal Antibody | CSB-RA977797A0HU | CD274 | Human | ELISA, WB, IHC |
| CD274 Recombinant Monoclonal Antibody | CSB-RA878942MA1HU | CD274 | Human | ELISA, IHC, FC |
| CD40 Recombinant Monoclonal Antibody | CSB-RA004936MA1HU | CD40 | Human | ELISA, IF, FC |
| CD44 Recombinant Monoclonal Antibody | CSB-RA004938A0HU | CD44 | Human | ELISA, WB, IHC |
| CD44 Recombinant Monoclonal Antibody | CSB-RA292372A0HU | CD44 | Human | ELISA, WB, IHC, IF, FC |
| CD44 Recombinant Monoclonal Antibody | CSB-RA004938MA1HU | CD44 | Human | ELISA, FC |
| CD47 Recombinant Monoclonal Antibody | CSB-RA802124A0HU | CD47 | Human | ELISA, WB, IHC, IF |
| CEACAM5 Recombinant Monoclonal Antibody | CSB-RA005165MA3HU | CEACAM5 | Human | ELISA, IF, FC |
| CEACAM5 Recombinant Monoclonal Antibody | CSB-RA005165MA1HU | CEACAM5 | Human | ELISA |
| CTLA4 Recombinant Monoclonal Antibody | CSB-RA213310A0HU | CTLA4 | Human | ELISA, IHC |
| CTLA4 Recombinant Monoclonal Antibody | CSB-RA006163MA1HU | CTLA4 | Human, Mouse | ELISA, WB, IF, FC |
| Phospho-EGFR (Y1092) Recombinant Monoclonal Antibody | CSB-RA007479A1092phHU | EGFR | Human | ELISA, WB |
| Phospho-EGFR (Y1068) Recombinant Monoclonal Antibody | CSB-RA007479A1068phHU | EGFR | Human | ELISA, WB |
| EGFR Recombinant Monoclonal Antibody | CSB-RA159341A0HU | EGFR | Human | ELISA, WB, IHC, IF, FC |
| EGFR Recombinant Monoclonal Antibody | CSB-RA794061A0HU | EGFR | Human | ELISA, WB, IHC |
| EGFR Recombinant Monoclonal Antibody | CSB-RA159341MA1HU | EGFR | Human | ELISA, IHC, FC |
| EPCAM Recombinant Monoclonal Antibody | CSB-RA932207A0HU | EPCAM | Human | ELISA, WB |
● ELISA試劑盒
藥物研發解決方案
專家講堂
參考文獻:
1. Cell 186, April 13, 2023
2. Larissa A. Korde, Mark R. Somerfield, Dawn L. Hershman, et al. Use of Immune Checkpoint Inhibitor Pembrolizumab in the Treatment of High-Risk, Early-Stage Triple-Negative Breast Cancer: ASCO Guideline Rapid Recommendation Update. DOI: 10.1200/JCO.22.00503 Journal of Clinical Oncology
3. Lajos Pusztai, Carsten Denkert, Joyce O'Shaughnessy, et al. Event-free survival by residual cancer burden after neoadjuvant pembrolizumab + chemotherapy versus placebo + chemotherapy for early TNBC: Exploratory analysis from KEYNOTE-522. J Clin Oncol 40, 2022 (suppl 16; abstr 503). DOI: 10.1200/JCO.2022.40.16_suppl.503
4. Breast cancer-WHO: https://www.who.int/news-room/fact-sheets/detail/breast-cancer