Notch信號(hào)通路在生物體內(nèi)扮演著至關(guān)重要的角色,不僅調(diào)控胚胎發(fā)育和成體組織穩(wěn)態(tài),更在多種癌癥的發(fā)生、發(fā)展中發(fā)揮雙重作用——既可作為腫瘤抑制因子,也能驅(qū)動(dòng)癌細(xì)胞增殖、血管生成及免疫逃逸。本專(zhuān)題頁(yè)面系統(tǒng)梳理Notch1–4受體與DLL/JAG配體的核心知識(shí),助您快速定位高品質(zhì)重組蛋白、抗體及ELISA試劑盒,推進(jìn)腫瘤及其他疾病領(lǐng)域的Notch相關(guān)研究。
Notch信號(hào)通路是進(jìn)化上高度保守的細(xì)胞間通訊系統(tǒng),最早因果蠅翅膀“缺刻”表型而被發(fā)現(xiàn)。在哺乳動(dòng)物中,該通路由4種跨膜受體(Notch1–4)和5種經(jīng)典配體(DLL1、DLL3、DLL4、JAG1、JAG2)組成,僅在相鄰細(xì)胞直接接觸時(shí)被激活,精準(zhǔn)調(diào)控細(xì)胞命運(yùn)決定、分化、增殖及組織穩(wěn)態(tài)。
所有Notch受體均為I型跨膜蛋白,在高爾基體中經(jīng)furin酶切后以異二聚體形式定位于細(xì)胞膜;配體同樣為跨膜蛋白,表達(dá)于鄰近細(xì)胞表面。當(dāng)配體與受體結(jié)合后,觸發(fā)兩次蛋白水解切割(ADAM介導(dǎo)的S2切割和γ-secretase介導(dǎo)的S3切割),釋放具有轉(zhuǎn)錄活性的Notch胞內(nèi)結(jié)構(gòu)域(NICD)。NICD進(jìn)入細(xì)胞核,與RBP-J等因子形成轉(zhuǎn)錄復(fù)合物,激活Hes/Hey等靶基因表達(dá),從而傳遞信號(hào)。
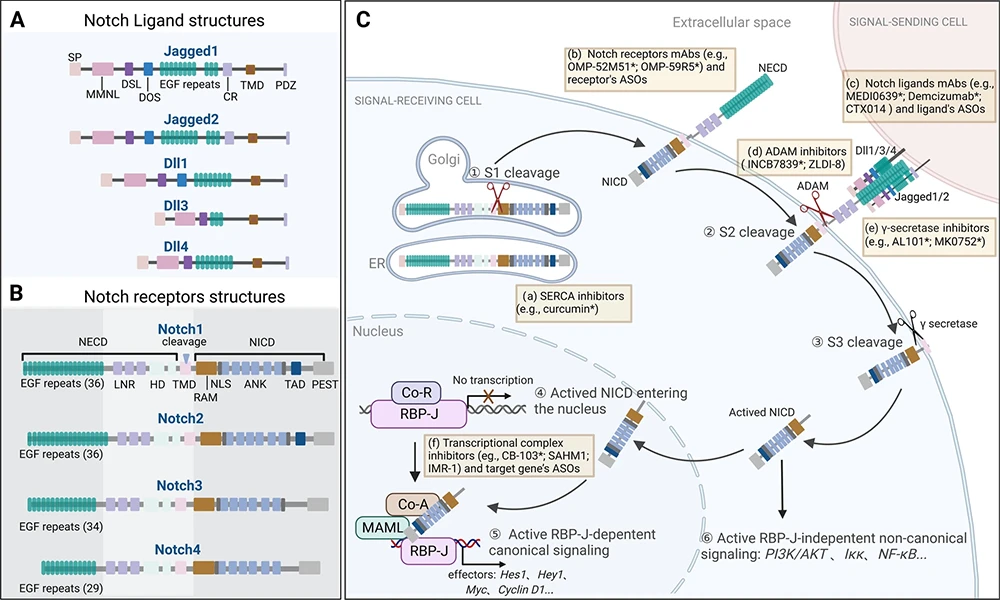
圖 NOTCH信號(hào)通路 [1]
(A. 配體結(jié)構(gòu)特征;B. 受體結(jié)構(gòu)特征;C. 信號(hào)接收與轉(zhuǎn)導(dǎo)過(guò)程。)
盡管通路機(jī)制高度相似,不同Notch受體與配體在組織分布、結(jié)合偏好及生物學(xué)功能上存在顯著差異——例如DLL4-Notch1主導(dǎo)血管生成,JAG1-Notch2參與腎臟發(fā)育,而DLL3則在小細(xì)胞肺癌中異常高表達(dá)。這些特異性決定了研究中靶點(diǎn)選擇與試劑匹配的重要性。
Notch信號(hào)通路的功能多樣性,源于其受體與配體在不同組織和發(fā)育階段的差異表達(dá),以及由此產(chǎn)生的信號(hào)輸出特異性。下表匯總了四種Notch受體與五種經(jīng)典配體的表達(dá)特征、核心生物學(xué)功能及相關(guān)的疾病背景,為您提供快速參考。
| 靶點(diǎn) | 主要表達(dá)部位 | 核心生物學(xué)功能 | 疾病關(guān)聯(lián) |
|---|---|---|---|
| Notch1 | 胸腺T細(xì)胞、血管內(nèi)皮、神經(jīng)前體細(xì)胞、乳腺上皮 | T細(xì)胞發(fā)育、血管生成、干細(xì)胞維持 | T細(xì)胞急性淋巴細(xì)胞白血病(T-ALL)[2,3]、乳腺癌 [4]、黑色素瘤 [5]、肝細(xì)胞癌 [6]、結(jié)直腸癌 [7]、阿爾茨海默病 [20] |
| Notch2 | 腎小管上皮、肝膽管細(xì)胞、B細(xì)胞、骨髓基質(zhì) | 腎臟/膽管發(fā)育、B細(xì)胞分化、肝再生 | 慢性淋巴細(xì)胞白血病(B-CLL )[8]、小細(xì)胞肺癌 [9]、胰腺癌 [10]、乳腺癌 [11]、Hajdu-Cheney綜合征 [18]、Alagille綜合征 [19] |
| Notch3 | 血管平滑肌細(xì)胞、腦小動(dòng)脈、周細(xì)胞 | 血管平滑肌穩(wěn)態(tài)、神經(jīng)血管功能 | 遺傳性腦小血管病(CADASIL)[12] |
| Notch4 | 血管內(nèi)皮、乳腺上皮 | 乳腺上皮發(fā)育、內(nèi)皮屏障功能 | 系統(tǒng)性硬皮病 [13]、黑色素瘤 [14]、肝細(xì)胞癌 [15]、哮喘 [16]、雙相情感障礙 [17] |
| 靶點(diǎn) | 主要表達(dá)部位 | 核心生物學(xué)功能 | 疾病關(guān)聯(lián) |
|---|---|---|---|
| DLL1 | 神經(jīng)干細(xì)胞、體節(jié)、造血微環(huán)境、腸隱窩 | 神經(jīng)發(fā)生、體節(jié)形成、造血干祖細(xì)胞調(diào)控 | 乳腺癌 [21]、神經(jīng)母細(xì)胞瘤 [22]、多發(fā)性骨髓瘤 [22] |
| DLL3 | 神經(jīng)內(nèi)分泌組織、胎兒腦、小細(xì)胞肺癌(SCLC) | 抑制經(jīng)典N(xiāo)otch信號(hào),調(diào)控神經(jīng)內(nèi)分泌命運(yùn) | 小細(xì)胞肺癌(SCLC) [24-26]、神經(jīng)內(nèi)分泌癌 [27] |
| DLL4 | 血管內(nèi)皮“尖端細(xì)胞”、腫瘤相關(guān)內(nèi)皮、胸腺上皮 | 血管出芽調(diào)控,限制內(nèi)皮“尖端細(xì)胞”過(guò)度增殖 | 結(jié)直腸癌 [28]、乳腺癌 [29]、肺癌 [30]、腎癌 [31]、胃癌 [32] |
| JAG1 | 肝膽管上皮、腎小管、心內(nèi)膜、肝星狀細(xì)胞、腫瘤相關(guān)成纖維細(xì)胞 | 器官發(fā)育(肝/腎/心)、免疫調(diào)節(jié)、纖維化 | Alagille綜合征 [33]、腎纖維化 [34]、三陰性乳腺癌 [35] |
| JAG2 | 骨細(xì)胞、胎盤(pán)滋養(yǎng)層細(xì)胞、T細(xì)胞、心臟瓣膜 | 骨發(fā)育、T細(xì)胞耐受 | 結(jié)直腸癌 [36]、多發(fā)性骨髓瘤 [37]、肺癌 [38] |
DLL3在超過(guò)80%的小細(xì)胞肺癌(SCLC)中高表達(dá),且?guī)缀醪辉谡=M織中出現(xiàn),使其成為理想的腫瘤特異性抗原。
深入閱讀:DLL3靶點(diǎn)新突破:再鼎醫(yī)藥公布ZL-1310早期臨床數(shù)據(jù)!
查看所有DLL3相關(guān)產(chǎn)品DLL4 在血管新生過(guò)程中發(fā)揮負(fù)調(diào)控作用——通過(guò)激活內(nèi)皮細(xì)胞上的 Notch1 受體,抑制過(guò)度出芽,維持血管網(wǎng)絡(luò)的有序性。然而,在多種實(shí)體瘤(如乳腺癌、結(jié)直腸癌、肝癌)中,DLL4 異常高表達(dá),導(dǎo)致腫瘤血管結(jié)構(gòu)紊亂、灌注不足,反而促進(jìn)缺氧、侵襲和免疫逃逸。
深入閱讀:DLL4:血管生成調(diào)控的關(guān)鍵靶點(diǎn)
查看所有DLL4相關(guān)產(chǎn)品Notch 信號(hào)通路在發(fā)育、穩(wěn)態(tài)及腫瘤等關(guān)鍵生物學(xué)過(guò)程中發(fā)揮核心調(diào)控作用,其深入研究離不開(kāi)高特異性、高可靠性的實(shí)驗(yàn)工具。華美生物提供覆蓋 Notch 全家族受體與配體的抗體、重組蛋白及 ELISA 試劑盒,助力您的科研精準(zhǔn)高效推進(jìn)。
貨號(hào):CSB-RA882142MA2HU
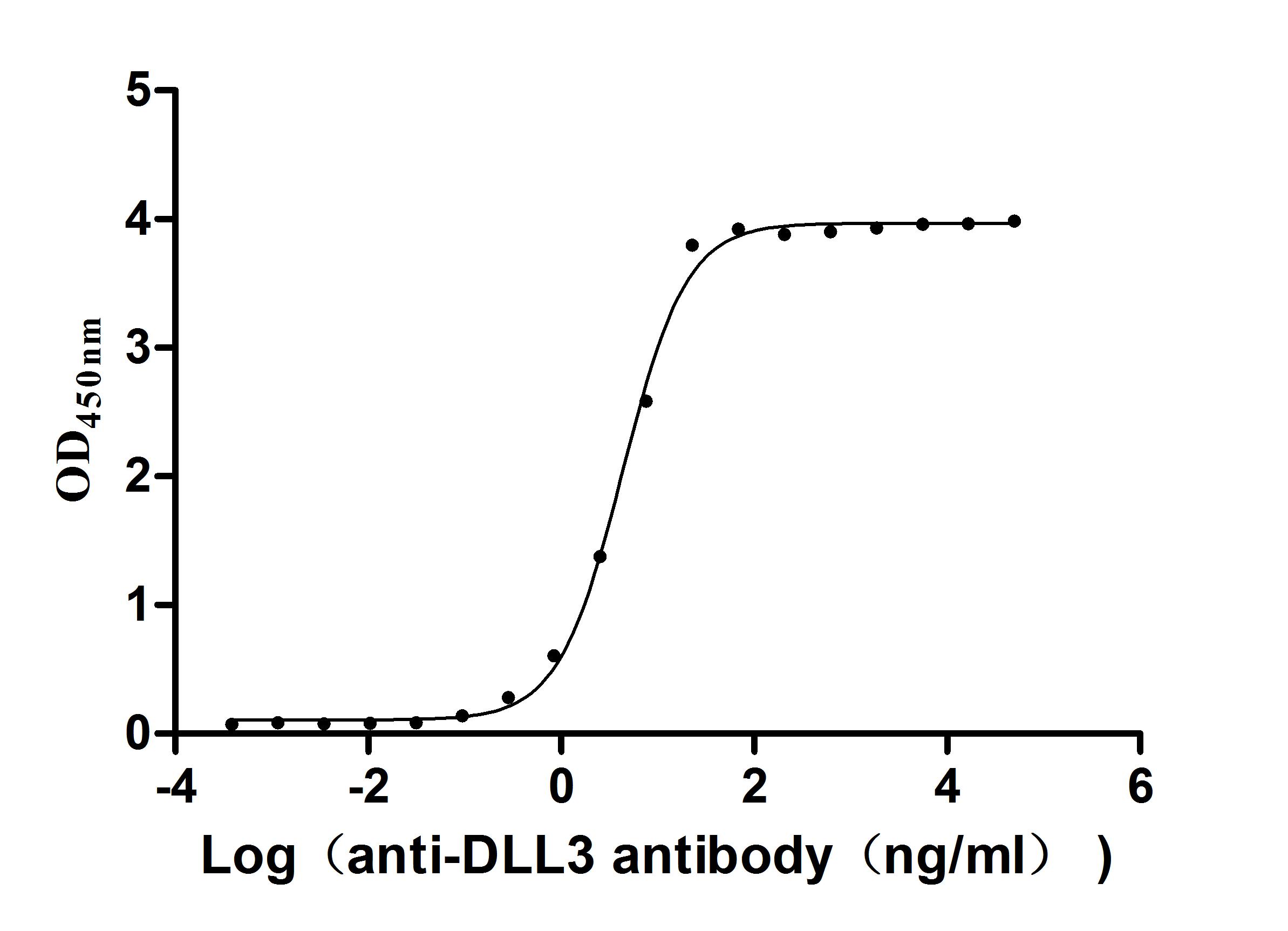
The Binding Activity of Human DLL3 with Anti-DLL3
recombinant antibody
Activity: Measured by its binding ability in a functional
ELISA. Immobilized Human DLL3 (CSB-MP882142HU2d7) at 2 μg/mL can bind Anti-DLL3
recombinant antibody. The EC50 is 3.990-4.723 ng/mL.
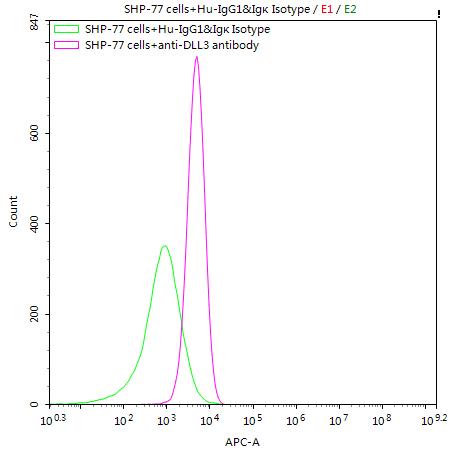
SHP-77 cells were stained with Human IgG1&Igκ Isotype Control (CSB-RA011156MA1HU)(green line) and anti-DLL3 antibody (CSB-RA882142MA2HU) (2μg/1*106cells) (red line), washed and then followed by APC-conjugated anti-Human IgG Fc antibody and analyzed with flow cytometry.
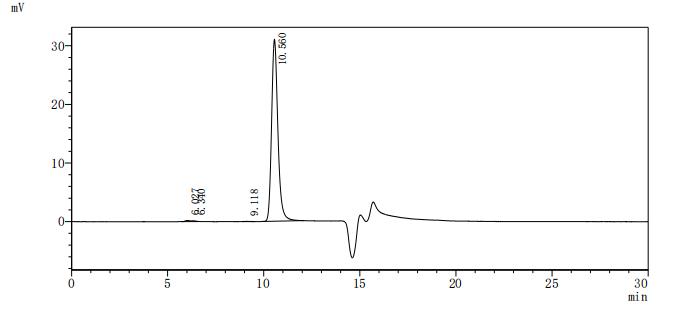
The purity of DLL3 was greater than 95% as determined by SEC-HPLC
貨號(hào):CSB-MP882142HU
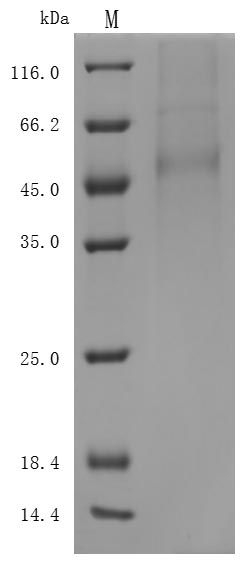
(Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
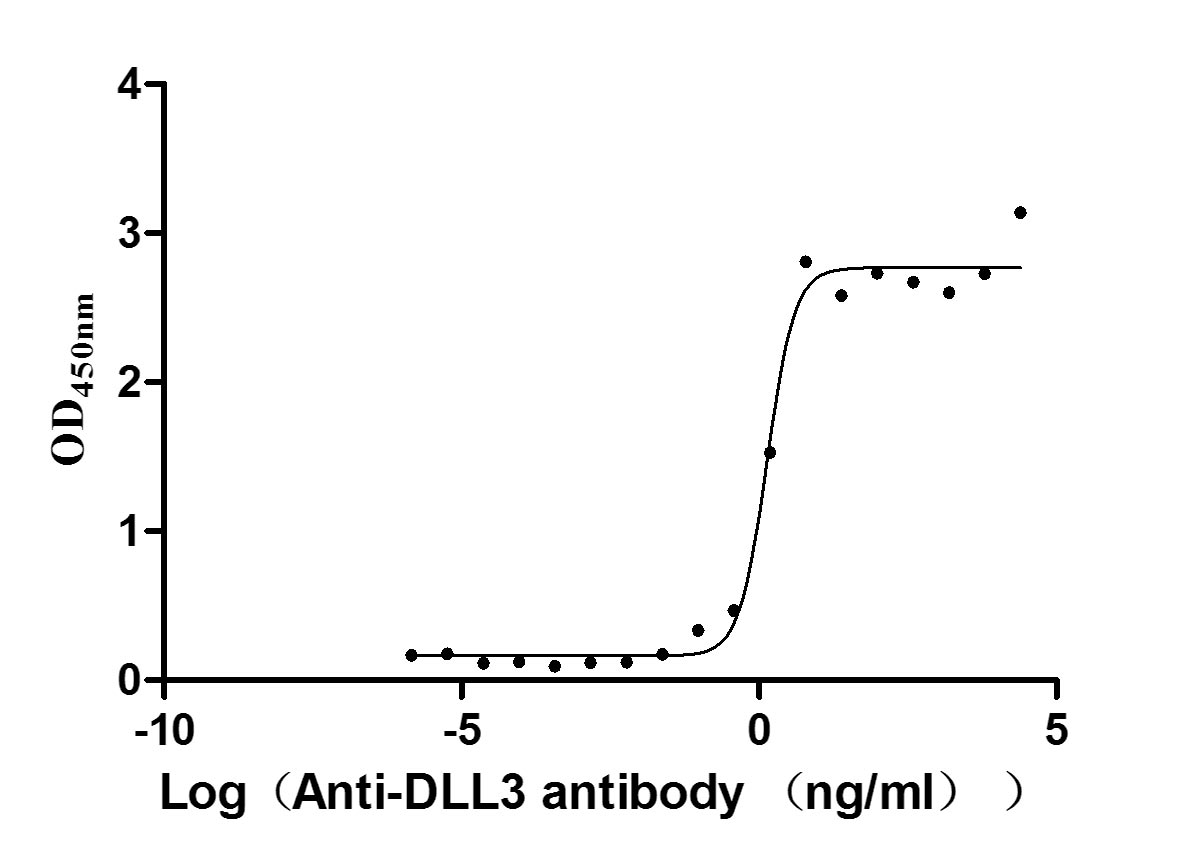
Activity: Measured by its binding ability in a functional ELISA. Immobilized DLL3 at 2 μg/ml can bind Anti-DLL3 Recombinant Antibody(CSB-RA882142A1HU), the EC50 is 1.102-1.707 ng/mL.
貨號(hào):CSB-EL006948HU
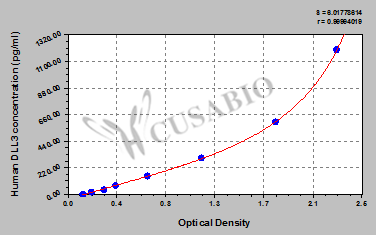
These standard curves are provided for demonstration only. A standard curve should be generated for each set of samples assayed.
| Target | Code | Product Name | Source |
|---|---|---|---|
| DLL3 | CSB-EP882142HU | Recombinant Human Delta-like protein 3 (DLL3), partial | E.coli |
| DLL3 | CSB-YP882142HU | Recombinant Human Delta-like protein 3 (DLL3), partial | Yeast |
| DLL3 | CSB-EP882142HU1 | Recombinant Human Delta-like protein 3 (DLL3), partial | E.coli |
| DLL3 | CSB-MP882142HU | Recombinant Human Delta-like protein 3 (DLL3), partial (Active) | Mammalian cell |
| DLL3 | CSB-MP3536MOV | Recombinant Macaca fascicularis Delta-like protein 3 (DLL3), partial (Active) | Mammalian cell |
| DLL3 | CSB-EP882142HU2-B | Recombinant Human Delta-like protein 3 (DLL3), partial, Biotinylated | E.coli |
| DLL3 | CSB-MP882142HU2 | Recombinant Human Delta-like protein 3 (DLL3), partial (Active) | Mammalian cell |
| DLL3 | CSB-MP882142HU2d7 | Recombinant Human Delta-like protein 3 (DLL3), partial (Active) | Mammalian cell |
| DLL3 | CSB-MP882142HU3 | Recombinant Human Delta-like protein 3 (DLL3), partial (Active) | Mammalian cell |
| DLL3 | CSB-MP882142HU3d7 | Recombinant Human Delta-like protein 3 (DLL3), partial (Active) | Mammalian cell |
| DLL4 | CSB-MP878862HU | Recombinant Human Delta-like protein 4 (DLL4), partial (Active) | Mammalian cell |
| DLL4 | CSB-MP7292MOV-C | Recombinant Macaca fascicularis Delta-like protein (DLL4), partial | Mammalian cell |
| Jag1 | CSB-EP870758MO | Recombinant Mouse Protein jagged-1 (Jag1), partial | E.coli |
| JAG1 | CSB-MP011927HUh6 | Recombinant Human Protein jagged-1 (JAG1), partial | Mammalian cell |
| NOTCH1 | CSB-EP015949HU | Recombinant Human Neurogenic locus notch homolog protein 1 (NOTCH1), partial | E.coli |
| NOTCH2NLB | CSB-BP3322HU | Recombinant Human Notch homolog 2 N-terminal-like protein B (NOTCH2NLB) | Baculovirus |
| NOTCH2NLB | CSB-EP3322HU | Recombinant Human Notch homolog 2 N-terminal-like protein B (NOTCH2NLB) | E.coli |
| Target | Code | Product Name | Tested Applications |
|---|---|---|---|
| DLL1 | CSB-PA006947GA01HU | DLL1 Antibody | ELISA, WB |
| DLL3 | CSB-RA882142MA2HU | DLL3 Recombinant Monoclonal Antibody | ELISA, FC |
| DLL3 | CSB-PA882142LA01HU | DLL3 Antibody | ELISA, IHC |
| DLL3 | CSB-PA882142LB01HU | DLL3 Antibody, HRP conjugated | ELISA |
| DLL3 | CSB-PA882142LC01HU | DLL3 Antibody, FITC conjugated | N/A |
| DLL3 | CSB-PA882142LD01HU | DLL3 Antibody, Biotin conjugated | ELISA |
| DLL4 | CSB-PA073786 | DLL4 Antibody | ELISA, IHC |
| DLL4 | CSB-PA831848 | DLL4 Antibody | ELISA, IHC |
| DLL4 | CSB-PA006949LA01HU | DLL4 Antibody | ELISA, IF |
| DLL4 | CSB-PA006949LB01HU | DLL4 Antibody, HRP conjugated | ELISA |
| DLL4 | CSB-PA006949LC01HU | DLL4 Antibody, FITC conjugated | N/A |
| DLL4 | CSB-PA006949LD01HU | DLL4 Antibody, Biotin conjugated | ELISA |
| JAG1 | CSB-RA272247A0HU | JAG1 Recombinant Monoclonal Antibody | ELISA, IHC |
| JAG1 | CSB-PA005638 | JAG1 Antibody | WB, ELISA |
| JAG1 | CSB-PA969766 | JAG1 Antibody | ELISA, IHC |
| JAG1 | CSB-PA234352 | JAG1 Antibody | ELISA, IHC |
| JAG1 | CSB-PA01949A0Rb | JAG1 Antibody | ELISA, IHC, IF |
| JAG1 | CSB-PA01949B0Rb | JAG1 Antibody, HRP conjugated | ELISA |
| JAG1 | CSB-PA01949C0Rb | JAG1 Antibody, FITC conjugated | N/A |
| JAG1 | CSB-PA01949D0Rb | JAG1 Antibody, Biotin conjugated | ELISA |
| Target | Code | Product Name | Detection Range | Sensitivity |
|---|---|---|---|---|
| DLL1 | CSB-EL006947HU | Human Delta-like protein 1(DLL1) ELISA kit | 31.25 pg/ml - 2000 pg/ml | 7.8 pg/ml |
| DLL3 | CSB-EL006948HU | Human Delta-like protein 3(DLL3) ELISA kit | 18.75 pg/mL-1200 pg/mL | 4.68 pg/mL |
| DLL4 | CSB-EL006949HU | Human Delta-like protein 4(DLL4) ELISA kit | 23.5 pg/mL-1500 pg/mL | 5.8 pg/mL |
| DLL4 | CSB-EL006949MO | Mouse Delta-like protein 4(DLL4) ELISA kit | 0.16 ng/ml-10 ng/ml | 0.04ng/ml |
| JAG1 | CSB-EL011927HU | Human Protein jagged-1(JAG1) ELISA kit | 0.156 ng/mL-10 ng/mL | 0.039 ng/mL |
| NOTCH1 | CSB-EL015949HU | Human Neurogenic locus notch homolog protein 1(NOTCH1) ELISA kit | 78 pg/mL-5000 pg/mL | 19.5 pg/mL |
| NOTCH3 | CSB-EL015952HU | Human Neurogenic locus notch homolog protein 3(NOTCH3) ELISA kit | 125 pg/mL-8000 pg/mL | 31.25 pg/mL |
[1] Li X,Yan X,Wang Y, et al. The Notch signaling pathway: a potential target for cancer immunotherapy. J Hematol Oncol. 2023;16 (1):45.
[2] Herranz D,Ambesi-Impiombato A,Palomero T, et al. A NOTCH1-driven MYC enhancer promotes T cell development, transformation and acute lymphoblastic leukemia. Nat Med. 2014;20 (10):1130-7.
[3] Bertulfo K,Perez-Duran P,Miller H, et al. Therapeutic targeting of the NOTCH1 and neddylation pathways in T cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2025;122 (14):e2426742122.
[4] Lei JH,Xu J,Lyu X, et al. NOTCH1 activation compensates BRCA1 deficiency and promotes triple-negative breast cancer formation. Nat Commun. 2020;11 (1):3256.
[5] Qiu H,Zmina PM,Huang AY, et al. Inhibiting Notch1 enhances immunotherapy efficacy in melanoma by preventing Notch1 dependent immune suppressive properties. Cancer Lett. 2018;434:144-151.
[6] Lindblad KE,Donne R,Liebling I, et al. NOTCH1 Drives Sexually Dimorphic Immune Responses in Hepatocellular Carcinoma. Cancer Discov. 2025;15 (3):495-510.
[7] Lu Y,Cao Y,Guo X, et al. Notch-Targeted Therapeutic in Colorectal Cancer by Notch1 Attenuation Via Tumor Microenvironment-Responsive Cascade DNA Delivery. Adv Healthc Mater. 2024;13 (22):e2400797.
[8] Hubmann R,Schwarzmeier JD,Shehata M, et al. Notch2 is involved in the overexpression of CD23 in B-cell chronic lymphocytic leukemia. Blood. 2002;99 (10):3742-7.
[9] Nayak R,Booker MA,Wang T, et al. Loss of NOTCH2 creates a TRIM28-dependent vulnerability in small cell lung cancer. Dev Cell. 2025;60 (24):3462-3479.e13.
[10] Xu J,Xu W,Yang X, et al. LncRNA MIR99AHG mediated by FOXA1 modulates NOTCH2/Notch signaling pathway to accelerate pancreatic cancer through sponging miR-3129-5p and recruiting ELAVL1. Cancer Cell Int. 2021;21 (1):674.
[11] Lee GH,Yoo KC,An Y, et al. FYN promotes mesenchymal phenotypes of basal type breast cancer cells through STAT5/NOTCH2 signaling node. Oncogene. 2018;37 (14):1857-1868.
[12] Heidari P,Taghizadeh M,Vakili O. Signaling pathways and molecular mechanisms involved in the onset and progression of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL); a focus on Notch3 signaling. J Headache Pain. 2025;26 (1):96.
[13] Cardinale CJ,Li D,Tian L, et al. Association of a rare NOTCH4 coding variant with systemic sclerosis: a family-based whole exome sequencing study. BMC Musculoskelet Disord. 2016;17 (1):462.
[14] Bonyadi Rad E,Hammerlindl H,Wels C, et al. Notch4 Signaling Induces a Mesenchymal-Epithelial-like Transition in Melanoma Cells to Suppress Malignant Behaviors. Cancer Res. 2016;76 (7):1690-7.
[15] Gramantieri L,Giovannini C,Lanzi A, et al. Aberrant Notch3 and Notch4 expression in human hepatocellular carcinoma. Liver Int. 2007;27 (7):997-1007.
[16] Harb H,Chatila TA. Recent patents in allergy and immunology: Method for treating asthma or allergic disease via anti-Notch4 mAb. Allergy.
[17] Li M,Su B. Up-regulation of NOTCH4 gene expression in bipolar disorder: future studies. Am J Psychiatry. 2013;170 (5):560-1.
[18] Simpson MA,Irving MD,Asilmaz E, et al. Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nat Genet. 2011;43 (4):303-5.
[19] McDaniell R,Warthen DM,Sanchez-Lara PA, et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79 (1):169-73.
[20] Brai E,Alina Raio N,Alberi L. Notch1 hallmarks fibrillary depositions in sporadic Alzheimer's disease. Acta Neuropathol Commun. 2016;4 (1):64.
[21] Singh S,Weindorfer C,Nandi A, et al. DLL1-responsive PD-L1 + tumor-associated macrophages promote endocrine resistance in breast cancer. Sci Transl Med. 2025;17 (823):eadr6207.
[22] Xu Y,Qiu Z,Chen J, et al. LINC00460 promotes neuroblastoma tumorigenesis and cisplatin resistance by targeting miR-149-5p/DLL1 axis and activating Notch pathway in vitro and in vivo. Drug Deliv Transl Res. 2024;14 (7):2003-2018.
[23] Xu D,Hu J,Xu S, et al. Dll1/Notch activation accelerates multiple myeloma disease development by promoting CD138+ MM-cell proliferation. Leukemia. 2012;26 (6):1402-5.
[24] Su PL,Chakravarthy K,Furuya N, et al. DLL3-guided therapies in small-cell lung cancer: from antibody-drug conjugate to precision immunotherapy and radioimmunotherapy. Mol Cancer. 2024;23 (1):97.
[25] Rudin CM,Reck M,Johnson ML, et al. Emerging therapies targeting the delta-like ligand 3 (DLL3) in small cell lung cancer. J Hematol Oncol. 2023;16 (1):66.
[26] Owen DH,Giffin MJ,Bailis JM, et al. DLL3: an emerging target in small cell lung cancer. J Hematol Oncol. 2019;12 (1):61.
[27] Hermans BCM,Derks JL,Thunnissen E, et al. DLL3 expression in large cell neuroendocrine carcinoma (LCNEC) and association with molecular subtypes and neuroendocrine profile. Lung Cancer. 2019;138:102-108.
[28] Naseri M,Saeednejad Zanjani L,Vafaei S, et al. Increased cytoplasmic expression of DLL4 is associated with favorable prognosis in colorectal cancer. Future Oncol. 2021;17 (24):3231-3242.
[29] Yan J,Xie Y,Liu Z, et al. DLL4-targeted CAR-T therapy sensitizes neoadjuvant chemotherapy via eliminating cancer stem cells and reshaping immune microenvironment in HER2 + breast cancer. J Immunother Cancer. 2024;12 (11):.
[30] Ding XY,Ding J,Wu K, et al. Cross-talk between endothelial cells and tumor via delta-like ligand 4/Notch/PTEN signaling inhibits lung cancer growth. Oncogene. 2012;31 (23):2899-906.
[31] Wang W,Hu W,Wang Y, et al. Long non-coding RNA UCA1 promotes malignant phenotypes of renal cancer cells by modulating the miR-182-5p/DLL4 axis as a ceRNA. Mol Cancer. 2020;19 (1):18.
[32] Afzalipour R,Abbasi-Dokht T,Sheikh M, et al. The Prediction of DLL4 as a Prognostic Biomarker in Patients with Gastric Cancer Using Anti-DLL4 Nanobody. J Gastrointest Cancer. 2024;55 (3):1380-1387.
[33] Gilbert MA,Keefer-Jacques E,Jadhav T, et al. Functional characterization of 2,832 JAG1 variants supports reclassification for Alagille syndrome and improves guidance for clinical variant interpretation. Am J Hum Genet. 2024;111 (8):1656-1672.
[34] Li G,Liu B,Yang H, et al. Omega-3 polyunsaturated fatty acids alleviate renal fibrosis in chronic kidney disease by reducing macrophage activation and infiltration through the JAG1-NOTCH1/2 pathway. Int Immunopharmacol. 2025;152:114454.
[35] Li C,Wang X,Shi D, et al. RFX5 promotes the progression of triple-negative breast cancer through transcriptional activation of JAG1. Hum Cell. 2025;38 (3):86.
[36] Vaish V,Kim J,Shim M. Jagged-2 (JAG2) enhances tumorigenicity and chemoresistance of colorectal cancer cells. Oncotarget. 2017;8 (32):53262-53275.
[37] Ghoshal P,Nganga AJ,Moran-Giuati J, et al. Loss of the SMRT/NCoR2 corepressor correlates with JAG2 overexpression in multiple myeloma. Cancer Res. 2009;69 (10):4380-7.
[38] Mandula JK,Sierra-Mondragon RA,Chang D, et al. Jagged2 targeting in lung cancer activates anti-tumor immunity via Notch-induced functional reprogramming of tumor-associated macrophages. Immunity. 2024;57 (5):1124-1140.e9.