IL-6:炎癥級聯反應重要參與者,功能最多、影響最廣泛的炎癥細胞因子之一!
日期:2024-04-11 09:59:36
2024年2月22日,Cell Reports雜志刊登了題為“Structural insights into IL-6 signaling inhibition by therapeutic antibodies”的文章,揭示了針對IL-6的抗體抑制劑Tocilizumab和Sarilumab,通過阻斷IL-6信號通路來,治療風濕性關節炎、細胞因子風暴和COVID-19肺炎等疾病的臨床應用前景 [1]。IL-6是一種單鏈糖蛋白細胞因子,由多種細胞產生,具有廣泛的生物學活性功能。大量的研究證實,IL-6在調節系統性炎癥反應綜合征、慢性自身免疫性疾病以及腫瘤發展等方面發揮著關鍵作用。目前,IL-6作為炎癥級聯反應重要參與者,是功能最多、影響最廣泛的炎癥細胞因子之一,正成為藥物研發的重要靶點!
1. 什么是IL-6?
1.1 IL-6的結構
白細胞介素-6(Interleukin-6,IL-6)是最早被發現的IL-6家族原型成員,其最初被確定為B細胞刺激因子-2(BSF-2)。IL-6分子量約為26 kDa,屬于磷酸化糖蛋白。人IL-6由212個氨基酸組成,其中包括28個氨基酸組成的信號肽序列,其基因定位于第7號染色體。IL-6的結構包含四個螺旋束,它們以“上-上-下-下”的拓撲結構排列,并且具有三個環狀結構(兩個較長的環,分別為A-B環和C-D環,以及一個較短的B-C環)。IL-6家族細胞因子包括IL-6、IL-11、IL-27、OSM、LIF、CNTF、CT1、CLCF1、IL-35、IL-39等10個成員。這些成員雖然在序列上并不相同,但它們都基于了共同的受體亞單位,即受體膜糖蛋白130(GP130),再通過與其配體結合,實現信號傳導 [1-7]。
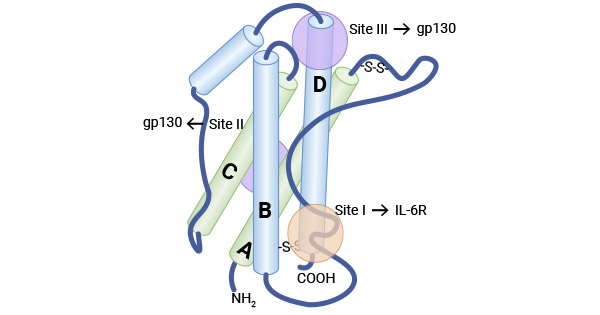
圖1. IL-6的結構 [3]
1.2 IL-6的表達和功能
IL-6是一種由免疫系統中多種細胞分泌的重要細胞因子,包括巨噬細胞和被感染的T細胞等。除了巨噬細胞外,還有許多其他細胞類型也能產生IL-6,如腸細胞、肝細胞、肺細胞等。IL-6在維持機體穩態中起著重要作用。當體內因感染或組織損傷而打破穩態時,IL-6會立即產生并通過對急性期反應和免疫應答的激活來對抗這些緊急壓力,從而有助于宿主防御。然而,IL-6過度合成及持續表達失調會導致病理性影響,包括急性期蛋白的產生、炎癥反應、免疫反應、宿主防御和造血等等。自從IL-6被發現以來,研究人員發現,IL-6可以與多種疾病密切關聯的現象,比如應激、肺炎、免疫、心血管以及腫瘤等 [1-7]。
2. IL-6的受體是什么?
IL-6結合沒有信號傳導能力的IL-6受體(IL-6R/IL-6Rα,也稱為CD126),進而結合第二個受體亞基130(GP130),形成了一個六聚體復合物,由2個配體分子、2個α亞基分子和2個β130分子組成。IL-6的受體有兩種類型(IL-6R和sIL-6R),它們分別啟動不同的信號轉導通路:膜受體啟動經典信號途徑;可溶性受體則啟動反式信號。兩種類型都需要GP130的參與。盡管GP130在細胞中廣泛表達,但IL-6受體/IL-6R僅存在于特定的細胞上,例如肝細胞、中性粒細胞、單核細胞、巨噬細胞以及T和B淋巴細胞等。可溶性IL-6R通過與IL-6形成復合物,這一過程介導了IL-6在表面僅含有GP130的多種細胞(如神經細胞、平滑肌細胞和內皮細胞)中的信號傳導 [8-10]。
3. IL-6相關的信號機制
IL-6通過其獨特的受體系統傳遞信號。它與IL-6R/IL-6Ra結合蛋白和信號轉導組分糖蛋白130(CD130)組成的細胞表面I型受體復合物相互作用。該復合物IL-6/IL-6R/GP130激活JAK/STAT3、PI3K/AKT/mTOR、RAS/RAF/MEK/ERK、YAP、SHP2/RAS/MAPK等信號通路的激活,也被稱為IL-6的經典信號轉導 [11]。
3.1 IL-6的經典信號轉導
在IL-6經典信號轉導中,關鍵蛋白包括Janus激酶(JAK)、STAT3以及Ras蛋白。IL-6激活JAK和STAT3,導致STAT3磷酸化并形成二聚體,隨后進入細胞核調節基因表達,促進細胞生長、分化和存活。此外,IL-6也激活Ras蛋白,進而增加MAPK活性,促進轉錄因子活性,參與細胞生長、免疫球蛋白合成等過程。另外,IL-6通過激活PI3K/PKB/Akt途徑調節信號傳導,影響細胞的生理活性 [10-11]。在未經刺激的CD4?T細胞中,IL-6能夠激活STAT3,進而誘導STAT3及其靶基因的轉錄,其中包括Arid5a,它保護STAT3 mRNA免受Regnase-1介導的降解作用。Arid5a對IL-6和STAT3 mRNA的調控對于IL-6的生成以及由IL-6受體介導的信號強度至關重要 [12]。
3.2 IL-6的反式信號傳導
IL-6僅與IL-6R結合,而不與GP130結合,因此IL-6R未表達的細胞對IL-6沒有反應。然而,膜結合的IL-6R可以被蛋白水解酶切割,釋放出與IL-6相互作用的可溶性IL-6R(sIL-6R),其主要的酶是ADAM17和ADAM10。因為GP130廣泛表達,這種sIL-6R的生成擴展了IL-6的作用范圍。sIL-6R結合GP130而不需要IL-6R的細胞進行信號傳導,這稱為IL-6的反式信號傳導。因此,IL-6的不同作用途徑調節著不同的生物效應,包括控制白細胞招募和腫瘤相關的炎性反應,在急性期免疫反應、造血功能和中樞平衡過程中起重要作用 [11]。
3.3 IL-6的其它信號轉導
在正常細胞中,IL-6的產生受到不同信號的調節,如IL-1、TNF、IFNs、DNA病毒、RNA病毒和細菌內毒素等 [13]。在急性炎癥中,單核細胞和巨噬細胞通過TLR激活IL-6的產生,而在慢性炎癥中,T細胞是IL-6主要來源之一。研究表明,多聚核苷酸Poly I:C能激活TLR3,從而誘導IL-6自我釋放,并通過STAT3磷酸化調節TLR2表達量 [14]。總之,IL-6在生理和病理過程中扮演復雜角色,針對性地阻斷IL-6及其信號通路已成為治療多種疾病研究的有效策略。
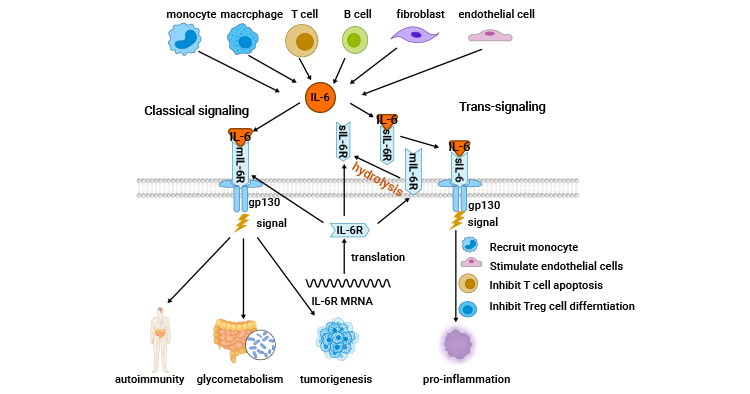
圖2. IL-6相關的信號機制 [11]
4. IL-6和疾病相關的研究
4.1 IL-6和炎癥研究
4.1.1 IL-6在急性炎癥中的研究
在炎癥早期,IL-6的合成和釋放是機體應對損傷或感染的重要反應之一。IL-6可以迅速被激活并釋放到局部組織,隨后通過血液循環迅速傳播到全身各個部位。在肝臟中,IL-6的產生可引發一系列生物學反應,其中包括急性期蛋白的合成,其中包括CRP、SAA和纖維蛋白原等。這些急性期蛋白的產生是機體對抗感染和修復組織損傷的關鍵步驟,它們不僅參與了免疫反應的調節,還有助于抵御病原體的入侵。通過調節炎癥反應的程度和持續時間,有助于維持組織內穩定的環境。因此,IL-6的早期釋放以及對急性期蛋白的合成對于抵御感染、修復組織損傷以及調節免疫反應至關重要 [15-18]。
4.1.2 IL-6在慢性炎癥中的研究
IL-6在慢性炎癥中扮演著一個多面手的角色。IL-6參與調節骨骼系統的穩態,對破骨細胞的分化與活化發揮著重要作用,進而影響骨質密度和結構,最終導致骨質疏松癥的發生 [19-20]。IL-6還能夠誘導血管內皮生長因子(VEGF)的過度產生,這會增加血管通透性,成為炎癥性疾病的典型特征之一。例如,在類風濕性關節炎的病理過程中,滑膜組織的血管通透性增加與IL-6誘導的VEGF過度表達密切相關,加劇了關節炎的病情 [21-22]。此外,IL-6的產生降低了白蛋白、纖連蛋白和轉鐵蛋白的生成。在慢性炎癥性疾病中,該過程可能導致嚴重的并發癥,如罕見病淀粉樣變性 [23]。因此,IL-6的多效性表現不僅僅局限于特定細胞類型,而是在整個炎癥性疾病過程中發揮著重要作用,其調節作用影響著骨骼健康和血管通透性等多個生理過程。
4.2 IL-6和神經系統研究
IL-6不僅在炎癥性疾病中發揮作用,近年來發現也在神經系統中發揮著重要作用。適量的IL-6在神經系統內調控神經元的發育、分化和存活過程,維持神經系統的生長和正常功能。然而,在遭受炎癥刺激或損傷時,機體會產生大量的IL-6。過剩的IL-6通過致炎作用進一步影響神經細胞,導致損傷。新生兒化膿性腦膜炎(NPM)是新生兒期常見的中樞神經系統感染性疾病之一。研究提示,在NPM患兒腦脊液中,細胞因子IL-6、IL-10濃度升高,提示其在NPM的發病機制中發揮一定作用 [24-25]。此外,IL-6水平在熱性驚厥患兒中明顯升高,尤其是復雜性熱性驚厥患兒。雖然IL-6基因存在多種位點,但其與熱性驚厥易感性之間的關系尚無一致結論 [26-28]。
4.3 IL-6在腫瘤晚期中的研究
IL-6在腫瘤晚期扮演重要角色,與其他IL-6家族細胞因子一樣,其在癌癥中的表達異常、受體信號失調,與不良臨床結果相關。IL-6直接影響癌細胞活動,間接調節基質細胞,影響腫瘤微環境。IL-6通過激活STAT3等信號通路參與多種致癌機制,包括增強癌細胞生長、血管生成、促進轉移和侵襲。在晚期,IL-6促進癌細胞的轉移和擴散,促進癌癥干細胞的增殖和種群擴張。其他IL-6家族成員如IL-11、LIF和OSM也與腫瘤生長相關。IL-6濃度與多種腫瘤的患病率、預后密切相關,可能成為腫瘤預后的獨立指標 [29-30]。
舉例來說,研究發現,結直腸腺瘤與血清IL-6濃度升高相關,晚期直腸癌患者的IL-6水平顯著高于早期患者,并且與患者的預后密切相關。然而,IL-6濃度不能單獨預測結直腸癌的狀態 [31];胃癌患者研究表明,胃黏膜中的IL-6水平與存活時間密切相關,提示IL-6可能是腫瘤侵襲的標志,具有預后價值 [32];肝癌組織中IL-6水平升高與不良預后相關,可能成為肝癌病人預后的獨立指標 [33];IL-6的分泌促使STAT3磷酸化,增強前列腺癌細胞的增殖和遷移能力,并同時抑制細胞的凋亡和上皮間質轉化(EMT) [34]。
4.4 IL-6和其它相關疾病研究
高水平的IL-6與冠心病風險增加相關,其作用可導致血管平滑肌細胞轉化為成骨樣細胞,促進血管內鈣鹽沉積 [35-36]。另一方面,IL-6具有極強的致炎敏感性和特異性,它可以促使滑膜和軟骨細胞釋放炎性遞質,從而減少滑膜炎癥反應,降低骨關節炎軟骨的損傷 [37]。同時,IL-6還能阻止蛋白聚糖和軟骨膠原的合成,有效抑制骨細胞的活性 [38-39]。IL-6還與胎糞吸入綜合征、睡眠呼吸暫停相關的肺動脈高壓等疾病密切相關,其相關作用機制需進一步研究 [40-42]。
一些研究也在探索靶向IL-6在其它病理過程中的具體作用機制,如參與內皮屏障功能障礙、心肌負性肌力效應、血管內皮生長因子誘導VE-cadherin磷酸化、以及在急性淋巴細胞白血病等惡性腫瘤治療中使用CAR-T細胞療法時所伴隨的細胞因子釋放綜合癥管理等方面的研究 [43-46]。同時指出IL-6信號在巨噬細胞替代激活途徑中限制內毒素血癥和肥胖相關胰島素抵抗方面的作用 [47-48]。此外,IL-6可促使產生鐵調節激素hepcidin,影響血液中的鐵和鋅水平,引發貧血和低鋅血癥 [49-50]。
5. IL-6的臨床研究前景
IL-6的臨床研究前景呈現出相當活躍和潛力。靶向IL-6的主要方法是使用IL-6受體(IL-6R)的單克隆抗體,目前已批準上市的藥物有3種,如托珠單抗(Tocilizumab)。Tocilizumab通過結合IL-6結合位點上的IL-6R來中和IL-6的活性,從而阻斷信號傳導。自托珠單抗應用于臨床治療以來,已成功治療多種疾病,如類風濕關節炎、系統性關節炎等,并表現出顯著的療效。當前,針對IL-6的治療策略已成功應用于若干慢性自身免疫性疾病,并有望在更多疾病的治療中得到廣泛應用。在藥物研發國家中,美國和中國占據了領先地位,在該賽道上是最具競爭力的國家,值得關注和重視。預計在未來十年內,IL-6抑制劑將廣泛應用于目前難以治療的各種疾病,包括細胞因子風暴,并有望克服這類疾病的難治性。綜上所述,該賽道的藥物研發進展較好,具有較高的可行性。
為鼎力協助各藥企針對IL6在系統性炎癥反應綜合征、慢性自身免疫性疾病以及腫瘤等在臨床中的研究,華美CUSABIO推出IL6(CSB-YP011664HU)活性蛋白產品,助力您在對IL6機制方面的研究或其潛在臨床價值的探索。
華美CUSABIO蛋白IL6
Recombinant Human Interleukin-6(IL6) (Active) Code: CSB-YP011664HU
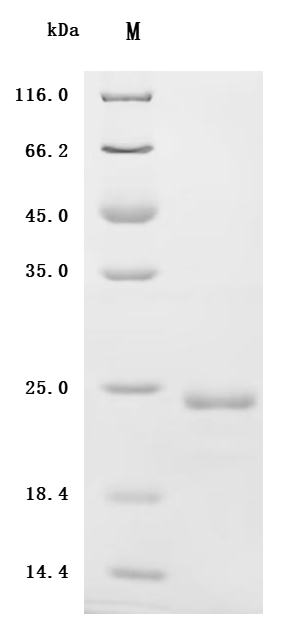
Purity was greater than 90% as determined by SDS-PAGE.
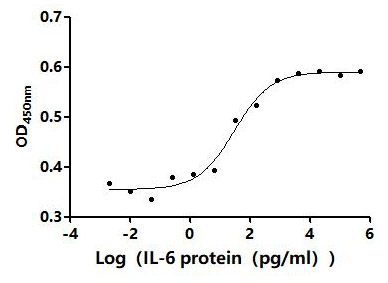
Immobilized Human IL6 at 2μg/mL can bind Anti-IL6 recombinant antibody (CSB-RA011664MA1HU). The EC50 is 35.80-41.82 ng/mL.
參考文獻:
[1] Wang, Mingxing, et al. "Structural insights into IL-6 signaling inhibition by therapeutic antibodies." Cell Reports 43.3 (2024).
[2] Tanaka, Toshio, Atsushi Ogata, and Masashi Narazaki. "Tocilizumab: an updated review of its use in the treatment of rheumatoid arthritis and its application for other immune-mediated diseases." Clinical Medicine Insights: Therapeutics 5 (2013): CMT-S9282.
[3] Mir, Manzoor Ahmad, Masrat Bashir, and Nusrat Jan. "The Role of Interleukin (IL)-6/IL-6 Receptor Axis in Cancer." Cytokine and Chemokine Networks in Cancer. Singapore: Springer Nature Singapore, 2023. 137-164.
[4] Yoshizaki, Kazuyuki, et al. "Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease." (1989): 1360-1367.
[5] Asaoku, Hideki, et al. "Decrease in BSF-2/IL-6 response in advanced cases of multiple myeloma." Blood 72.2 (1988): 429-432.
[6] Tanaka, Toshio, Masashi Narazaki, and Tadamitsu Kishimoto. "IL-6 in inflammation, immunity, and disease." Cold Spring Harbor perspectives in biology 6.10 (2014): a016295.
[7] Unver, Nese, and Florencia McAllister. "IL-6 family cytokines: Key inflammatory mediators as biomarkers and potential therapeutic targets." Cytokine & growth factor reviews 41 (2018): 10-17.
[8] Mihara, Masahiko, et al. "IL-6/IL-6 receptor system and its role in physiological and pathological conditions." Clinical science 122.4 (2012): 143-159.
[9] Rafiq, Sajjad, et al. "A common variant of the interleukin 6 receptor (IL-6r) gene increases IL-6r and IL-6 levels, without other inflammatory effects." Genes & Immunity 8.7 (2007): 552-559.
[10] Heo, Tae-Hwe, Joseph Wahler, and Nanjoo Suh. "Potential therapeutic implications of IL-6/IL-6R/gp130-targeting agents in breast cancer." Oncotarget 7.13 (2016): 15460.
[11] Xu, Junnv, et al. "IL-6/STAT3 is a promising therapeutic target for hepatocellular carcinoma." Frontiers in oncology 11 (2021): 760971.
[12] Masuda, Kazuya, et al. "Arid5a controls IL-6 mRNA stability, which contributes to elevation of IL-6 level in vivo." Proceedings of the National Academy of Sciences 110.23 (2013): 9409-9414.
[13] Wang, Qing, et al. "Soluble interleukin-6 receptor-mediated innate immune response to DNA and RNA viruses." Journal of virology 87.20 (2013): 11244-11254.
[14] Liu, Xin, et al. "IL‐6 expression promoted by Poly (I: C) in cervical cancer cells regulates cytokine expression and recruitment of macrophages." Journal of cellular and molecular medicine 24.3 (2020): 2284-2293.
[15] Yoshizaki, Kazuyuki. "Pathogenic role of IL-6 combined with TNF-α or IL-1 in the induction of acute phase proteins SAA and CRP in chronic inflammatory diseases." Advances in TNF Family Research: Proceedings of the 12th International TNF Conference, 2009. New York, NY: Springer New York, 2010.
[16] Yap, S. H., et al. "Tumor necrosis factor (TNF) inhibits interleukin (IL)-1 and/or IL-6 stimulated synthesis of C-reactive protein (CRP) and serum amyloid A (SAA) in primary cultures of human hepatocytes." Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1091.3 (1991): 405-408.
[17] Hagihara, Keisuke, et al. "IL-6 plays a critical role in the synergistic induction of human serum amyloid A (SAA) gene when stimulated with proinflammatory cytokines as analyzed with an SAA isoform real-time quantitative RT-PCR assay system." Biochemical and biophysical research communications 314.2 (2004): 363-369.
[18] Fu, Yang, et al. "The use of PCT, CRP, IL-6 and SAA in critically ill patients for an early distinction between candidemia and Gram positive/negative bacteremia." Journal of Infection 64.4 (2012): 438-440.
[19] Manolagas, Stavros C., Teresita Bellido, and Robert L. Jilka. "New insights into the cellular, biochemical, and molecular basis of postmenopausal and senile osteoporosis: roles of IL-6 and gp130." International journal of immunopharmacology 17.2 (1995): 109-116.
[20] Edwards, C. J., and E. Williams. "The role of interleukin-6 in rheumatoid arthritis-associated osteoporosis." Osteoporosis international 21 (2010): 1287-1293.
[21] Yoo, Seung-Ah, et al. "Arginine-rich anti-vascular endothelial growth factor (anti-VEGF) hexapeptide inhibits collagen-induced arthritis and VEGF-stimulated productions of TNF-α and IL-6 by human monocytes." The Journal of Immunology 174.9 (2005): 5846-5855.
[22] Cheng, Wen-Xiang, et al. "Genistein inhibits angiogenesis developed during rheumatoid arthritis through the IL-6/JAK2/STAT3/VEGF signalling pathway." Journal of orthopaedic translation 22 (2020): 92-100.
[23] Okuda, Yasuaki. "AA amyloidosis–Benefits and prospects of IL-6 inhibitors." Modern rheumatology 29.2 (2019): 268-274.
[24] Liu, Chunmei, et al. "The value of interleukin-6 (IL-6) within 6 hours after birth in the prompt diagnosis of early-onset neonatal sepsis." Translational Pediatrics 9.5 (2020): 629.
[25] Li, Jing, et al. "Relationship between PCT, IL-6 and HS-CRP in serum and cerebrospinal fluid and prognosis of neonates with purulent meningitis." (2021): 2831-2836.
[26] Straussberg, Rachel, et al. "Pro-and anti-inflammatory cytokines in children with febrile convulsions." Pediatric neurology 24.1 (2001): 49-53.
[27] Saghazadeh, Amene, et al. "Proinflammatory and anti-inflammatory cytokines in febrile seizures and epilepsy: systematic review and meta-analysis." Reviews in the Neurosciences 25.2 (2014): 281-305.
[28] Gupta, Surbhi, et al. "Serum interleukin-6 levels in children with febrile seizures." Indian Pediatrics 55 (2018): 411-413.
[29] Taher, Mustafa Yassin, David Marc Davies, and John Maher. "The role of the interleukin (IL)-6/IL-6 receptor axis in cancer." Biochemical Society Transactions 46.6 (2018): 1449-1462.
[30] Gonzalez-Aparicio, Manuela, and Carlos Alfaro. "Implication of interleukin family in cancer pathogenesis and treatment." Cancers 13.5 (2021): 1016.
[31] Waldner, Maximilian J., Sebastian Foersch, and Markus F. Neurath. "Interleukin-6-a key regulator of colorectal cancer development." International journal of biological sciences 8.9 (2012): 1248.
[32] Ashizawa, Tatsuto, et al. "Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor." Gastric Cancer 8 (2005): 124-131.
[33] He, Guobin, et al. "Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling." Cell 155.2 (2013): 384-396.
[34] Nguyen, Daniel P., Jinyi Li, and Ashutosh K. Tewari. "Inflammation and prostate cancer: the role of interleukin 6 (IL‐6)." BJU international 113.6 (2014): 986-992.
[35] Kurozumi, Akira, et al. "IL-6 and sIL-6R induces STAT3-dependent differentiation of human VSMCs into osteoblast-like cells through JMJD2B-mediated histone demethylation of RUNX2." Bone 124 (2019): 53-61.
[36] Anderson, Daniel R., et al. "IL-6 and its receptors in coronary artery disease and acute myocardial infarction." Cytokine 62.3 (2013): 395-400.
[37] Latourte, Augustin, et al. "Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis." Annals of the rheumatic diseases 76.4 (2017): 748-755.
[38] Flannery, Carl R., et al. "IL-6 and its soluble receptor augment aggrecanase-mediated proteoglycan catabolism in articular cartilage." Matrix Biology 19.6 (2000): 549-553.
[39] Sui, Yihong, et al. "Mechanical injury potentiates proteoglycan catabolism induced by interleukin‐6 with soluble interleukin‐6 receptor and tumor necrosis factor α in immature bovine and adult human articular cartilage." Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 60.10 (2009): 2985-2996.
[40] Steiner, M. Kathryn, et al. "Interleukin-6 overexpression induces pulmonary hypertension." Circulation research 104.2 (2009): 236-244.
[41] Haakonsen Lindenskov, Paal Helge, et al. "Meconium aspiration syndrome: possible pathophysiological mechanisms and future potential therapies." Neonatology 107.3 (2015): 225-230.
[42] Huiguo, Liu, et al. "The change of interleukin-6 and tumor necrosis factor in patients with obstructive sleep apnea syndrome." Journal of Tongji Medical University 20 (2000): 200-202.
[43] Desai, Tina R., et al. "Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway." Journal of surgical research 104.2 (2002): 118-123.
[44] Comini, Laura, et al. "Acute haemodynamic effects of IL-6 treatment in vivo: involvement of vagus nerve in NO-mediated negative inotropism." Cytokine 30.5 (2005): 236-242.
[45] Yang, Yong-Chang, et al. "Interleukin-6 downregulates the expression of vascular endothelial-cadherin and increases permeability in renal glomerular endothelial cells via the trans-signaling pathway." Inflammation 45.6 (2022): 2544-2558.
[46] Zhang, Yinqiang, et al. "Timing of tocilizumab administration under the guidance of IL-6 in CAR-T therapy for R/R acute lymphoblastic leukemia." Frontiers in Immunology 13 (2022): 914959.
[47] Mauer, Jan, et al. "Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin." Nature immunology 15.5 (2014): 423-430.
[48] Kuo, Feng-Chih, et al. "Circulating soluble IL-6 receptor concentration and visceral adipocyte size are related to insulin resistance in Taiwanese adults with morbid obesity." Metabolic Syndrome and Related Disorders 15.4 (2017): 187-193.
[49] Nemeth, Elizabeta, et al. "IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin." The Journal of clinical investigation 113.9 (2004): 1271-1276.
[50] Liuzzi, Juan P., et al. "Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response." Proceedings of the National Academy of Sciences 102.19 (2005): 6843-6848.










