轉鐵蛋白受體TFR1(TFRC):鐵穩態關鍵成員,貧血、神經退行性疾病、癌癥新銳靶點!
日期:2024-10-23 16:01:00
轉鐵蛋白受體TFR1是介導鐵離子進入細胞通道的關鍵成員之一,在調節細胞鐵代謝和維持鐵平衡中發揮關鍵作用。癌細胞為了快速增殖需要大量鐵,導致細胞表面轉鐵蛋白受體1(TfR1)顯著上調,TfR1通過與攜鐵蛋白轉鐵蛋白結合來介導鐵的攝取。利用這一現象和 TfR1 的快速內吞速率,美國丹娜-法伯癌癥研究所Xin Zhou課題組開發了轉鐵蛋白受體靶向嵌合體(TransTAC),這是一種用于膜蛋白降解的異雙特異性抗體模式 [1]。TransTAC被設計用于驅動感興趣的靶蛋白與TfR1從細胞表面共同內吞,并促使靶蛋白進入溶酶體降解途徑。這一研究于2024年9月25日發表在Nature上,TransTAC代表了一類有前景的新型雙功能抗體家族,可用于精確調控膜蛋白和靶向癌癥治療。
現有研究證實,TFR1在許多腫瘤細胞中高表達,是潛在的腫瘤標志物,且針對TFR1進行治療可以有效地抑制腫瘤生長和轉移。此外,TFR1還與其它疾病如貧血、鐵代謝障礙性疾病等有關。因此,以TFR1為靶點的治療策略來靶向調節細胞內鐵水平,可以在相關疾病的臨床應用中發揮重要作用。
1. 什么是TFR1?
1.1 TFR1的結構
轉鐵蛋白受體1(Transferrin receptor protein 1,TFR1)也被稱為CD71或TFRC。TFR1/TFRC是一種II型跨膜蛋白,是調節細胞內鐵元素轉運過程的最重要膜蛋白。TFR1是由兩個同源二聚體的亞基通過二硫鍵交聯而成。每個單體包含一個大的胞外C端區域,一個單跨膜區域及一個短的N端區域。C端區域作為外功能區,包含了與轉鐵蛋白(Transferrin,Tf)相結合的位點(圖1) [2-4]。目前已發現兩種轉鐵蛋白受體,分別是TFR1和TFR2,它們在結構和功能上都比較相似。在正常生理條件下,TFR1與轉鐵蛋白Tf發生相互作用,促進鐵的吸收。這種結合形式是血液中鐵的主要存在方式 [5-6]。
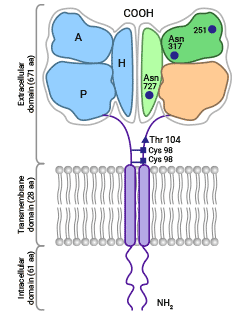
圖1. TFR1的結構 [2]
1.2 TFR1的表達
TFR1是一種廣泛表達于人體幾乎所有細胞和組織類型中的膜蛋白。TFR1在人體需要鐵元素時發揮作用,介導鐵離子的轉運和代謝。TFR1的表達廣泛分布于免疫系統、造血系統(如骨髓干細胞、紅細胞和白細胞)、神經系統(如神經元和神經膠質細胞)、生殖系統、心臟、肝臟、腎臟等各種組織和細胞類型。TFR1的表達水平受多種因素影響,包括細胞內鐵含量、細胞分化狀態、激素調節以及炎癥狀態等 [7-9]。
1.3 TFR1的功能
TFR1最主要的生理功能是與轉鐵蛋白(transferrin,Tf)結合,通過內吞方式介導細胞對鐵的攝取。因此,Tf-TFR1系統被認為是機體獲取鐵離子的重要途徑。具體而言,首先,Tf和鐵離子(Fe3+或Fe2+形式)結合后,其空間結構隨之發生相應變化,將鐵離子包入蛋白內形成Tf-Fe2+。其次,TFR1在生理pH下與Tf-Fe2+結合,Tf-TFR1復合物被網格蛋白(Clathrin)通過小窩的內吞作用內部化(圖2) [10-12]。
隨之,胞內Tf-TFR1復合物被運輸至內體酸化,TFR1和Tf的氨基酸殘基相互作用,引起構象改變促使鐵離子釋放,TFR1通過高爾基復合體循環至細胞表面完成鐵離子運輸。總之,TFR1在細胞和組織中扮演著重要角色,通過TFR1的調節來平衡細胞內鐵含量,維持人體鐵穩態是保證人體各項生理機能正常運作的必要條件 [10-12]。
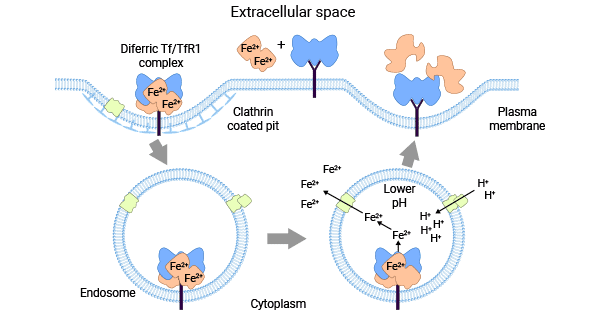
圖2. Tf-TFR1系統平衡細胞內鐵含量 [2]
2. TFR1相關的調控機制
TFR1是細胞最重要的鐵元素攝取因子。TFR1表達量下降或異常會導致細胞缺鐵,而過多的鐵則可能催化活性氧(ROSs)并損傷生物大分子。為了確保充足的鐵元素同時避免其毒性,細胞已經發展出多種機制來調控TFR1表達水平。盡管TFR1異常表達在多種疾病中發揮作用,但其分子機制和作用仍未完全明確。因此,還需要更深入地研究和探索。
TFR1的表達受多種刺激條件調控。在轉錄水平中,當細胞發生缺氧時,缺氧誘導因子(HIF)和其他轉錄因子如c-Myc、GATA1、Ets-1以及促紅細胞生成素Stat5可以促進其轉錄。TFR1的轉錄后水平主要由IRP1和IRP2調控,它們與TFR1 mRNA中的IRE結合來影響基因表達 [13-15]。
在翻譯后水平,CD133(PROM1)是TFR1轉運鐵元素過程中的負調節因子,同時EGF受體、c-Abl分子和MARCH8分子可能也參與其中。如下圖所示,一項研究揭示FLCN有可能在翻譯、或者翻譯后水平調控TFR1的表達,即Tf-TFR1復合物可與含有Rab11蛋白的循環內體相結合,回到細胞膜上(圖3)[13-17]。
TFR1在疾病中發揮調控作用。例如,在膠質瘤中,TFR1通過炎癥反應,細胞周期,DNA損失及DNA甲基化等機制參與膠質瘤的發生發展。此外,PD1信號,如IL17,IL18,NF-kβ,FOXM1,FOCAL及JAK-STAT信號可能是TFR1調控的關鍵信號通路 [18-20]。在神經干細胞中敲除TFR1發現,條件性敲除小鼠有癲癇的癥狀。并且在此研究中還發現GluA2在海馬神經元突觸上表達增加,其中突觸前的神經遞質釋放能力下降,突觸后長時程增強(LTP)受到一定的抑制 [21-23]。
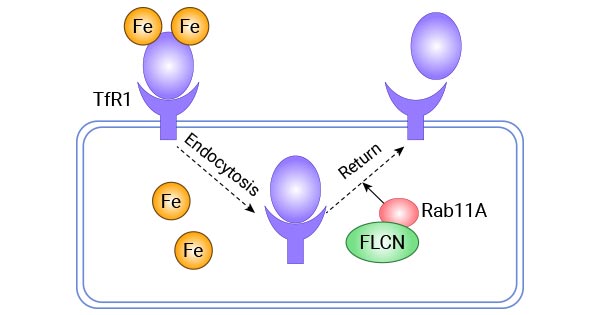
圖3. FLCN通過Rab11A調控Tf-TFR1蛋白的回收運輸 [17]
3. TFR1在腫瘤、神經退行性等疾病中的作用
正常人體鐵代謝處于平衡狀態,當其中的某一調控環節出現異常導致平衡被打破時,會影響細胞內自由基形成并加速氧化應激反應進展,同時也可導致腫瘤發生和發展。TFR1作為細胞攝取鐵元素過程中最重要的調控受體,在多種疾病的發生發展過程中起到重要作用。
3.1 TFR1和腫瘤
現有研究表明TFR1在甲狀腺癌 [24]、食管鱗狀細胞癌 [25]、乳腺癌 [26]、肝癌 [27]、結腸癌 [28]、白血病 [29]、肺癌 [30]、胰腺癌 [31]、鼻咽癌 [31]等惡性腫瘤中顯著表達。但在部分惡性腫瘤中,TFR1表達情況尚不明確,其中包括:前列腺癌、睪丸癌等。例如,在肝癌中,TFR1在肝癌中的表達與甲胎蛋白和血清凝血酶原的濃度有關 [27];在乳腺癌中,敲降IRP2表達,可提高鐵蛋白重鏈的表達,并下調TFR1蛋白表達,從而抑制乳腺癌細胞的生長 [26, 32];在結腸癌中,TFR1的高表達可激活IL-6/IL-11-Stat3信號通路,促進結腸上皮細胞的增殖和凋亡,從而加重結腸黏膜的損傷并導致結腸癌的發生 [28, 33]。
3.2 TFR1和神經退行性疾病
鐵代謝的紊亂是引發神經退行性疾病的病理生理機制之一。鐵在大腦中的蓄積,與阿爾茨海默病、帕金森病、肌萎縮側索硬化等神經退行性疾病有關 [34]。阿爾茨海默病是最常見神經退行性疾病之一,其主要以淀粉樣斑塊的積聚以及某些神經元的丟失為發病特征。有研究表明,抑制阿爾茲海默模型小鼠大腦顳葉皮層中的鐵攝取蛋白TFR1、TF以及DMT1的表達,可有效緩解鐵過載狀態 [35-36]。
3.3 TFR1和貧血
TFR1與Tf復合物的結合,對紅細胞生成過程中細胞獲取鐵元素有著重要意義。當人體內發生缺鐵或者紅細胞生成增多時,TFR1的表達將被反應性上調。臨床研究證實,地中海貧血小鼠體內的溶性轉鐵蛋白受體(sTFR1)和TF的水平都顯著高于正常值。進一步研究揭示,TFR1在β-地中海紅系前體細胞中異常高表達,降低TFR1的表達可有效調節貧血小鼠中無效紅細胞的生成并改善小鼠的貧血以及鐵過載情況 [7, 37]。
3.4 TFR1和其它疾病
TFR1不僅參與細胞鐵離子運輸,有研究提示TFR1還可作為多種病毒受體介導HCV與宿主細胞膜融合,在HCV入胞過程中發揮重要作用。丙型肝炎病毒(HCV)是導致慢性化肝炎、原發性肝癌的主要病原體。因此,TFR1作為HCV抗病毒靶點的潛在可能性值得關注 [38-40]。在神經元中,研究發現TFR1對mGlul的轉運起到重要的調節作用,并且可能參與了mGlul信號通路,且對小腦的運動協調能力起到影響 [41-42]。
4. TFR1的臨床研究進展
目前已有多款針對轉鐵蛋白受體1(TFR1)的臨床藥物正在研發中,藥物類型涵蓋抗體核酸偶聯藥物、雙體、單體、抗體融合蛋白、ADC藥物等;適應癥涉及非小細胞肺癌、食管癌、黏多醣貯積癥型、肌強直性營養不良、阿爾茨海默癥、杜氏肌營養不良癥等。其中,JCR Pharmaceuticals Co., Ltd.的Pabinafusp Alfa,一款靶向IDS和TfR1的抗體融合蛋白已于2021年日本上市。近年來,基于TFR1的靶向治療策略在不斷發展。有研究利用TFR1提高抗體跨越血腦屏障的轉運能力,并與抗β-淀粉樣肽單抗相結合形成特異性復合抗體,以提高阿爾茨海默病患者的治療效果 [43-44]。
同時,抗TFRC的抗體JST-TFR09和抗TFRC單克隆抗體A24分別可抑制腫瘤細胞對鐵元素的攝取,以及誘導T系細胞白血病中惡性細胞的凋亡 [45-46]。這些研究提示,TFR1可成為有效的靶標分子參與到多種疾病的臨床治療。未來,隨著TFR1相關研究的不斷深入和完善,有望為患者提供更加精準、有效的治療,為他們帶來更多的獲益和新希望。
5. 華美生物TFR1相關產品
● TFR1重組蛋白
Recombinant Human Transferrin receptor protein 1(TFRC),partial (Active) (Code: CSB-MP3648HU)
High specificity was validated by SDS-PAGE. SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
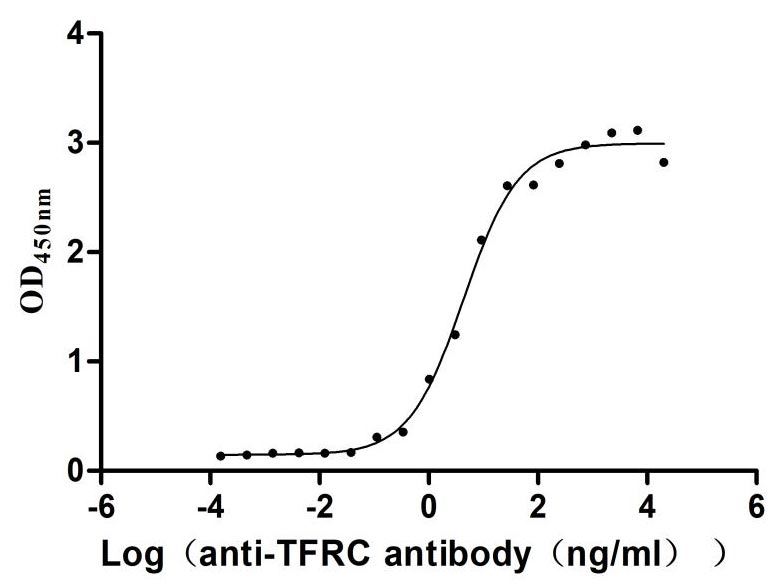
Immobilized Human TFRC at 2μg/mL can bind Anti-TFRC recombinant antibody (CSB-RA023441MA1HU), the EC50 is 3.305-8.220 ng/mL.
● TFR1抗體
| 產品名稱 | 適應種屬 | 應用 | 貨號 |
|---|---|---|---|
| TFRC Recombinant Monoclonal Antibody | Human | ELISA | CSB-RA023441MA1HU |
| TFRC Antibody | Human | ELISA, IHC, IF | CSB-PA07219A0Rb |
| TFRC Antibody | Human, Mouse | ELISA, WB, IHC | CSB-PA001477 |
| TFRC Antibody | Human | ELISA, WB | CSB-PA005607 |
| Phospho-TFRC (S24) Antibody | Human, Mouse | ELISA, WB, IHC | CSB-PA010092 |
| TFRC Antibody | Human, Mouse, Rat | ELISA, WB, IHC | CSB-PA023441GA01HU |
| TFRC Antibody | Human, Mouse, Rat | ELISA, WB, IHC | CSB-PA104885 |
| TFRC Antibody | Human, Mouse, Rat | ELISA, WB, IHC | CSB-PA834425 |
● TFR1 ELISA試劑盒
| 產品名稱 | 檢測樣本 | 檢測范圍 | 貨號 |
|---|---|---|---|
| Mouse transferrin receptor,TFR ELISA Kit | serum, plasma, tissue homogenates | 0.625 ng/mL-40 ng/mL | CSB-E08389m |
| Human soluble transferrin receptor,sTfR ELISA Kit | serum, plasma | Request Information | CSB-E09100h |
參考文獻:
[1] Transferrin receptor targeting chimeras for membrane protein degradation. Nature, 2024.
[2] Candelaria, Pierre V., et al. "Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents." Frontiers in immunology 12 (2021): 607692.
[3] Jabara, Haifa H., et al. "A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency." Nature genetics 48.1 (2016): 74-78.
[4] Greene, Christopher J., et al. "Transferrin receptor 1 upregulation in primary tumor and downregulation in benign kidney is associated with progression and mortality in renal cell carcinoma patients." Oncotarget 8.63 (2017): 107052.
[5] Kawabata, Hiroshi. "Transferrin and transferrin receptors update." Free Radical Biology and Medicine 133 (2019): 46-54.
[6] Kleven, Mark D., Shall Jue, and Caroline A. Enns. "Transferrin receptors TfR1 and TfR2 bind transferrin through differing mechanisms." Biochemistry 57.9 (2018): 1552-1559.
[7] Li, Huihui, et al. "Decreasing TfR1 expression reverses anemia and hepcidin suppression in β-thalassemic mice." Blood, The Journal of the American Society of Hematology 129.11 (2017): 1514-1526.
[8] Magro, Gaetano, et al. "Aberrant expression of TfR1/CD71 in thyroid carcinomas identifies a novel potential diagnostic marker and therapeutic target." Thyroid 21.3 (2011): 267-277.
[9] Silvestri, Laura, et al. "The extrahepatic role of TFR2 in iron homeostasis." Frontiers in pharmacology 5 (2014): 93.
[10] Tang, Li-Jing, et al. "Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion." Free Radical Biology and Medicine 162 (2021): 339-352.
[11] Kawabata, Hiroshi. "Transferrin and transferrin receptors update." Free Radical Biology and Medicine 133 (2019): 46-54.
[12] Nadadur, S. S., K. Srirama, and Anuradha Mudipalli. "Iron transport & homeostasis mechanisms: their role in health & disease." Indian Journal of Medical Research 128.4 (2008): 533-544.
[13] Gammella, Elena, et al. "The transferrin receptor: the cellular iron gate." Metallomics 9.10 (2017): 1367-1375.
[14] Tsiftsoglou, Asterios S., Ioannis S. Vizirianakis, and John Strouboulis. "Erythropoiesis: model systems, molecular regulators, and developmental programs." IUBMB life 61.8 (2009): 800-830.
[15] Bayeva, Marina. Novel Regulators of Mitochondrial and Cellular Iron Homeostasis. Diss. Northwestern University, 2012.
[16] Zhao, Lingling, et al. "FLCN is a novel Rab11A-interacting protein that is involved in the Rab11A-mediated recycling transport." Journal of Cell Science 131.24 (2018): jcs218792.
[17] Wang, Xiaojuan, et al. "FLCN regulates transferrin receptor 1 transport and iron homeostasis." Journal of Biological Chemistry 296 (2021).
[18] Ge, Xiaogang, et al. "Treatment with paraquat affects the expression of ferroptosis-related genes." Human & Experimental Toxicology 42 (2023): 09603271231167585.
[19] Wu, Hongrong, et al. "Identification and validation of transferrin receptor protein 1 for predicting prognosis and immune infiltration in lower grade glioma." Frontiers in Molecular Neuroscience 15 (2022).
[20] Wu, Hongrong, et al. "Identification and validation of transferrin receptor protein 1 for predicting prognosis and immune infiltration in lower grade glioma." Frontiers in Molecular Neuroscience 15 (2022): 972308.
[21] Klüssendorf, Malte, et al. "The Golgi-associated PDZ domain protein Gopc/PIST is required for synaptic targeting of mGluR5." Molecular Neurobiology 58.11 (2021): 5618-5634.
[22] Zhou, Jia-Huan, et al. "Ablation of TFR1 in Purkinje cells inhibits mGlu1 trafficking and impairs motor coordination, but not autistic-like behaviors." Journal of Neuroscience 37.47 (2017): 11335-11352.
[23] Warming, Hannah Kate. Haemoglobin neurotoxicity, haptoglobin scavenging and synaptic function in subarachnoid haemorrhage. Diss. University of Southampton, 2023.
[24] Parenti, Rosalba, Lucia Salvatorelli, and Gaetano Magro. "Anaplastic thyroid carcinoma: current treatments and potential new therapeutic options with emphasis on TfR1/CD71." International journal of endocrinology 2014 (2014).
[25] Ye, Jiecheng, et al. "A novel iron (II) phenanthroline complex exhibits anticancer activity against TFR1-overexpressing esophageal squamous cell carcinoma cells through ROS accumulation and DNA damage." Biochemical Pharmacology 166 (2019): 93-107.
[26] Corte-Rodriguez, Mario, et al. "Quantitative analysis of transferrin receptor 1 (TfR1) in individual breast cancer cells by means of labeled antibodies and elemental (ICP-MS) detection." Analytical chemistry 91.24 (2019): 15532-15538.
[27] Xiao, Chong, et al. "Transferrin receptor regulates malignancies and the stemness of hepatocellular carcinoma-derived cancer stem-like cells by affecting iron accumulation." PLoS One 15.12 (2020): e0243812.
[28] Cui, Can, et al. "Downregulation of TfR1 promotes progression of colorectal cancer via the JAK/STAT pathway." Cancer Management and Research 11 (2019): 6323.
[29] Liu, Qian, et al. "Significance of CD71 expression by flow cytometry in diagnosis of acute leukemia." Leukemia & lymphoma 55.4 (2014): 892-898.
[30] Jeong, Seung Min, Sunsook Hwang, and Rho Hyun Seong. "Transferrin receptor regulates pancreatic cancer growth by modulating mitochondrial respiration and ROS generation." Biochemical and biophysical research communications 471.3 (2016): 373-379.
[31] Martínez;nez, Laura E., et al. "Targeting TfR1 with the ch128. 1/IgG1 Antibody Inhibits EBV-driven Lymphomagenesis in Immunosuppressed Mice Bearing EBV+ Human Primary B-cells." Molecular cancer therapeutics 20.9 (2021): 1592-1602.
[32] Chen, Chunli, et al. "Deferoxamine-induced high expression of TfR1 and DMT1 enhanced iron uptake in triple-negative breast cancer cells by activating IL-6/PI3K/AKT pathway." OncoTargets and therapy 12 (2019): 4359.
[33] Huang, Luji, et al. "Iron metabolism in colorectal cancer." Frontiers in Oncology 13 (2023).
[34] Whitnall, Megan, and Des R. Richardson. "Iron: a new target for pharmacological intervention in neurodegenerative diseases." Seminars in pediatric neurology. Vol. 13. No. 3. WB Saunders, 2006.
[35] Yu, Xiaojun, et al. "Decreased iron levels in the temporal cortex in postmortem human brains with Parkinson disease." Neurology 80.5 (2013): 492-495.
[36] Lu, Li-Na, et al. "Expression of iron transporters and pathological hallmarks of Parkinson’s and Alzheimer’s diseases in the brain of young, adult, and aged rats." Molecular neurobiology 54 (2017): 5213-5224.
[37] Cabrera, C., et al. "Relationship between iron deficiency and expression of genes involved in iron metabolism in human myocardium and skeletal muscle." International journal of cardiology 379 (2023): 82-88.
[38] Fillebeen, Carine, and Kostas Pantopoulos. "Hepatitis C virus infection causes iron deficiency in Huh7. 5.1 cells." PLoS One 8.12 (2013): e83307.
[39] Lindenbach, Brett D., and Charles M. Rice. "The ins and outs of hepatitis C virus entry and assembly." Nature Reviews Microbiology 11.10 (2013): 688-700.
[40] Bonkovsky, Herbert L., et al. "Iron and HFE or TfR1 mutations as comorbid factors for development and progression of chronic hepatitis C." Journal of Hepatology 37.6 (2002): 848-854.
[41] Kalinowska, Magdalena. Metabotropic regulation of dendritic spine structural plasticity. Diss. Yeshiva University, 2015.
[42] Zhou, Jia-Huan, et al. "Ablation of TFR1 in Purkinje cells inhibits mGlu1 trafficking and impairs motor coordination, but not autistic-like behaviors." Journal of Neuroscience 37.47 (2017): 11335-11352.
[43] Bray, Natasha. "Transferrin'bispecific antibodies across the blood–brain barrier." Nature Reviews Drug Discovery 14.1 (2015): 14-15.
[44] Pardridge, William M. "Blood–brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody." Expert opinion on drug delivery 12.2 (2015): 207-222.
[45] Shimosaki, Shunsuke, et al. "Development of a complete human IgG monoclonal antibody to transferrin receptor 1 targeted for adult T-cell leukemia/lymphoma." Biochemical and biophysical research communications 485.1 (2017): 144-151.
[46] Candelaria, Pierre V., et al. "Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents." Frontiers in immunology 12 (2021): 607692.










