Recombinant Human Heat shock protein HSP 90-alpha (HSP90AA1)
-
中文名稱:人HSP90AA1重組蛋白
-
貨號:CSB-YP010802HU
-
規格:
-
來源:Yeast
-
其他:
-
中文名稱:人HSP90AA1重組蛋白
-
貨號:CSB-EP010802HU
-
規格:
-
來源:E.coli
-
其他:
-
中文名稱:人HSP90AA1重組蛋白
-
貨號:CSB-EP010802HU-B
-
規格:
-
來源:E.coli
-
共軛:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名稱:人HSP90AA1重組蛋白
-
貨號:CSB-BP010802HU
-
規格:
-
來源:Baculovirus
-
其他:
-
中文名稱:人HSP90AA1重組蛋白
-
貨號:CSB-MP010802HU
-
規格:
-
來源:Mammalian cell
-
其他:
產品詳情
-
純度:>85% (SDS-PAGE)
-
基因名:
-
Uniprot No.:
-
別名:Heat shock 86 kDa; Heat shock protein 90kDa alpha cytosolic class A member 1; Heat shock protein 90kDa alpha cytosolic class B member 1; Heat shock protein HSP 90 alpha ; Heat shock protein HSP 90 beta; Heat shock protein HSP 90-alpha; HS90A_HUMAN; HSP 84; HSP 86; Hsp 90; HSP86; HSP90A; HSP90AA1; HSP90AB1; HSP90B; HSPC1; HSPC2; HSPCAL1 ; HSPCAL4; Renal carcinoma antigen NY-REN-38
-
種屬:Homo sapiens (Human)
-
蛋白長度:Full Length of Mature Protein
-
表達區域:2-732
-
氨基酸序列PEETQTQDQ PMEEEEVETF AFQAEIAQLM SLIINTFYSN KEIFLRELIS NSSDALDKIR YESLTDPSKL DSGKELHINL IPNKQDRTLT IVDTGIGMTK ADLINNLGTI AKSGTKAFME ALQAGADISM IGQFGVGFYS AYLVAEKVTV ITKHNDDEQY AWESSAGGSF TVRTDTGEPM GRGTKVILHL KEDQTEYLEE RRIKEIVKKH SQFIGYPITL FVEKERDKEV SDDEAEEKED KEEEKEKEEK ESEDKPEIED VGSDEEEEKK DGDKKKKKKI KEKYIDQEEL NKTKPIWTRN PDDITNEEYG EFYKSLTNDW EDHLAVKHFS VEGQLEFRAL LFVPRRAPFD LFENRKKKNN IKLYVRRVFI MDNCEELIPE YLNFIRGVVD SEDLPLNISR EMLQQSKILK VIRKNLVKKC LELFTELAED KENYKKFYEQ FSKNIKLGIH EDSQNRKKLS ELLRYYTSAS GDEMVSLKDY CTRMKENQKH IYYITGETKD QVANSAFVER LRKHGLEVIY MIEPIDEYCV QQLKEFEGKT LVSVTKEGLE LPEDEEEKKK QEEKKTKFEN LCKIMKDILE KKVEKVVVSN RLVTSPCCIV TSTYGWTANM ERIMKAQALR DNSTMGYMAA KKHLEINPDH SIIETLRQKA EADKNDKSVK DLVILLYETA LLSSGFSLED PQTHANRIYR MIKLGLGIDE DDPTADDTSA AVTEEMPPLE GDDDTSRMEE VD
-
蛋白標簽:Tag?type?will?be?determined?during?the?manufacturing?process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
產品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
復溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
儲存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事項:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相關產品
靶點詳情
-
功能:Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle that is linked to its ATPase activity which is essential for its chaperone activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function. Engages with a range of client protein classes via its interaction with various co-chaperone proteins or complexes, that act as adapters, simultaneously able to interact with the specific client and the central chaperone itself. Recruitment of ATP and co-chaperone followed by client protein forms a functional chaperone. After the completion of the chaperoning process, properly folded client protein and co-chaperone leave HSP90 in an ADP-bound partially open conformation and finally, ADP is released from HSP90 which acquires an open conformation for the next cycle. Plays a critical role in mitochondrial import, delivers preproteins to the mitochondrial import receptor TOMM70. Apart from its chaperone activity, it also plays a role in the regulation of the transcription machinery. HSP90 and its co-chaperones modulate transcription at least at three different levels. In the first place, they alter the steady-state levels of certain transcription factors in response to various physiological cues(PubMed:25973397). Second, they modulate the activity of certain epigenetic modifiers, such as histone deacetylases or DNA methyl transferases, and thereby respond to the change in the environment. Third, they participate in the eviction of histones from the promoter region of certain genes and thereby turn on gene expression. Binds bacterial lipopolysaccharide (LPS) and mediates LPS-induced inflammatory response, including TNF secretion by monocytes. Antagonizes STUB1-mediated inhibition of TGF-beta signaling via inhibition of STUB1-mediated SMAD3 ubiquitination and degradation. Mediates the association of TOMM70 with IRF3 or TBK1 in mitochodria outer membrane which promotes host antiviral response.
-
基因功能參考文獻:
- RPAP3 provides a flexible scaffold for coupling HSP90 to the human R2TP co-chaperone complex. PMID: 29662061
- The study shows that a conserved tryptophan in the middle domain senses the interaction of Hsp90 with a stringent client protein and transfers this information via a cation-pi interaction with a neighboring lysine. PMID: 29662162
- While activation in c-Src is strictly controlled by ATP-binding and phosphorylation, the authors find that activating conformational transitions are spontaneously sampled in Hsp90-dependent Src mutants. PMID: 28290541
- chemotherapy agents can induce HSP90AA1 expression in osteosarcoma cells. And HSP90AA1, acting as an important regulator of autophagy, is a critical factor in the development of osteosarcoma chemoresistance both in vitro and in vivo. HSP90AA1 provides a novel therapeutic target for improving osteosarcoma treatment. PMID: 30153855
- We confirm that miR-628-3p promotes apoptosis and inhibits migration in A549 cells by negatively regulating HSP90. Our results may reveal a novel strategy for lung cancer treatment PMID: 29888262
- Data recognize HSP90 as a novel binding partner of PKM2 in hepatocellular carcinoma (HCC) cells. HSP90 potentiates the glycolysis and proliferation, reduces the apoptosis and thus enhances the growth of HCC cells through PKM2 Thr-328 phosphorylation maintaining its stability. PMID: 29262861
- EGFR expression stratified most pronounced among HSP90low tumours, where the EGFRhigh phenotype was associated with longer survival PMID: 28765916
- the SGT1-HSP90 complex contributes to the E3 ligase activity of the CUL4A complex that is necessary for CENP-A ubiquitylation and CENP-A deposition at the centromere. PMID: 28816574
- our data suggested that Hsp90alpha could positively regulate the self-renewal of BCSCs by facilitating the nuclear translocation of c-Myc and EZH2 to maintain BMI1 expression. PMID: 28914785
- HSP90 contributes to cutaneous vasodilation via NOS-dependent mechanisms in young habitually active men during exercise in the heat. PMID: 28751373
- The association between the MEEVD C-terminal peptide from the heat shock protein 90 (Hsp90) and tetratricopeptide repeat A (TPR2A) domain of the heat shock organizing protein (Hop) is a useful prototype to study the fundamental molecular details about the Hop-Hsp90 interaction. Observed are conformational changes of the peptide and the protein receptor induced by binding. The binding free energy is 8.4 kcal/mol. PMID: 28723223
- Our findings demonstrate Hsp90 blockade leads to ICN1 destabilization, providing an alternative strategy to antagonize oncogenic Notch1 signaling with Hsp90-selective inhibitors PMID: 28143869
- Generated multiple mutant KRAS-driven cancer cell lines with acquired resistance to the purine-scaffold HSP90 inhibitor PU-H71. Report a Y142N missense mutation in the ATP-binding domain of HSP90alpha that co-occurred with amplification of the HSP90AA1 locus in resistant cells. PMID: 28032595
- ATM is the primary kinase responsible for phosphorylation of Hsp90alpha after exposure ionizing radiation. PMID: 27738310
- molecular modeling was employed to incorporate experimental data using partial constructs of the Hsp90 C-terminal domain. PMID: 27771574
- findings suggested that this mechanism may be exploited by the Hsp90-Cdc37 chaperone to recruit and protect intrinsically dynamic kinase clients from degradation PMID: 29267381
- The findings establish an active role for Tsc1 as a facilitator of Hsp90-mediated folding of kinase and non-kinase clients-including Tsc2-thereby preventing their ubiquitination and proteasomal degradation. PMID: 29127155
- Data indicate HSP90 inhibitors as a class of preferred drugs for treatment combination with immunotherapy. PMID: 28878208
- Data suggest that SOCS3 is an important signaling protein in CLL, and Hsp90 inhibitors represent an approach to target transcriptional repression in B cell lymphoproliferative disorders. PMID: 27107422
- FKBP51 is primarily localized in mitochondria and hTERT is totally nuclear, upon the onset of oxidative stress, FKBP51 (but not FKBP52) becomes mostly nuclear colocalizing with hTERT, and longer exposure times to peroxide favors hTERT export to mitochondria. PMID: 27233944
- High HSP90 expression is associated with Colorectal Cancers. PMID: 28870917
- High HSP90 expression is associated with prostate cancer. PMID: 28038472
- Data suggest HSP90AA1-dependent regulation of ATM-NBN-CHK2 and ATR-CHK1 axes influences cells capability to repair double-stranded DNA damage; mechanisms include phosphorylation, polyubiquitination, and proteasomal degradation/proteolysis. (HSP90AA1 = heat shock protein 90kDa alpha; ATM = ataxia telangiectasia mutated protein; NBN = nibrin; CHK = checkpoint kinase; ATR = ataxia telangiectasia and Rad3 related kinase) PMID: 28631426
- Data show that pyruvate kinase M2 (PKM2) directly interacted with mutant growth factor receptor (EGFR) and heat-shock protein 90 (HSP90), and thus stabilized EGFR by maintaining its binding with HSP90 and co-chaperones. PMID: 26500058
- Binding of FM807 to the N-terminus of Hsp90 disrupted Hsp90/client complexes, resulting in degradation of the Hsp90 client protein EGFR and inhibition of the downstream pathway. PMID: 28157708
- Conventional as well as scaled molecular dynamics simulations further demonstrate that citrullination of selected Arg residues leads to progressive disruption of HSP90 tertiary structure, promoting exposure of R502/R510 to PAD modification and subsequent autoantibody binding. PMID: 27448590
- SYK is an HSP90 client protein, and B-cell receptor signaling-dependent phosphorylation of HSP90 on Y197 is required for this interaction. HSP90 promotes Burkitt lymphoma cell survival by maintaining tonic B-cell receptor signaling. PMID: 28064214
- Data indicate a chaperone function of nicotinamide mononucleotide adenylyl transferase 2 (NMNAT2), independent from its enzymatic activity, and NMNAT2 complexes with heat shock protein 90 (HSP90) to refold aggregated protein substrates. PMID: 27254664
- In the bound state, the Hsp90 dimer predominantly populates an open conformation, and transthyretin retains its globular structure. PMID: 28218749
- CD30 facilitates phosphorylation of heat shock factor 1, activates heat shock promoter element, and induces heat shock protein (HSP) 90. PMID: 27870927
- However, once the mumps virus L protein formed a mature polymerase complex with the P protein, Hsp90 activity was no longer required for the stability and activity of the L protein. PMID: 28053100
- HSP90 may be essential for stabilization and function of P2X7Rs through an action on the cysteine-rich domain of the cytoplasmic the C-terminus. PMID: 27301716
- HSP90AA1 and AB1 genes exhibit low expression in breast cancers highly sensitive to chemotherapy and may indicate the patients with higher probability of pathological complete response. PMID: 28051275
- the effect of HSP90 inhibition on IL-17-mediated cytokine and antimicrobial peptide expression in keratinocytes following heat treatment, was examined. PMID: 27279135
- epididymis secretory protein 4 had better specificity than CA125 in discriminating ovarian cancer, and endometrial cancer from benign gynecological diseases in southern China population PMID: 27302312
- Hsp90 has roles in the regulation of autophagy, such as toll-like receptor (TLR)-mediated autophagy, Ulk1-mediated mitophagy, and chaperone-mediated autophagy (CMA) [review] PMID: 26432328
- this study identified HSP90AA1 as a new potential biomarker for Behcet's disease by comparing highly ranked genes from all the built network-derived gene lists, which was confirmed the with real-world clinical samples PMID: 27226232
- Data show that heat shock protein 90 (HSP90) inhibitor 17-DMAG caused loss of ret proto-oncogene protein (RET) and proto-oncogene protein erbB-3 (ERBB3) phosphorylation and lead to rapid cell death. PMID: 26595521
- Hsp103 associates with cochaperone proteins, such as Hop, Cdc37 and Aha1, similar to Hsp90. The extra domain reduces the ATP hydrolysis when compared to Hsp90 thereby acting as a negative regulator of the chaperones intrinsic ATPase activity. PMID: 23951259
- Data suggest that synergistic mechanism between heat shock protein 90 (Hsp90) inhibitor SNX-7081 and fludarabine nucleoside (2-FaraA) may provide an alternative treatment for chronic lymphocytic leukemia (CLL) patients with p53 protein mutations. PMID: 26556860
- The expression of HSP90A was increased in the HCC cells, serum, and tissues. Immunohistochemistry analysis on 76 clinical tissue samples also suggested the relevance between HSP90A expression and HCC metastatic behavior. PMID: 26704341
- Aarsd1 inhibits the activity of a paradigmatic Hsp90 client protein. PMID: 26884463
- study confirmed Hsp90 as an influenza virus A PB2 polymerase interacting protein, and established that Hsp90 interacts with both the E627 and 627K variants, but has established this interaction is species independent, and both mammalian and avian Hsp90 can bind to the PB2 protein PMID: 26616658
- Data show that high-affinity heat shock protein 90 (HSP90) binding conferred by the inhibitor backbone could be exploited for conjugate accumulation within tumor cells. PMID: 26271675
- In conjunction with HSP90, the cytoplasmic USP19 may play a key role in triage decision for the disease-related polyQ-expanded substrates, suggesting a function of USP19 in quality control of misfolded proteins by regulating their protein levels PMID: 26808260
- The region of aa 250-295 of BGLF4 is essential for the BGLF4/Hsp90 interaction. PMID: 26982469
- The thermodynamics of binding of Cyp-40 to Hsp90 shows remarkable temperature sensitivity in the physiological temperature range. PMID: 26330616
- HSP90 overexpression is a prognostic marker for cholangiocarcinoma. HSP90-targeted therapy may be an option for a subset of cholangiocarcinoma. PMID: 26141945
- From our screening methodology, we identified HCAb2 as a breast tumor specific heavy chain antibody targeting cell surface heat shock protein 90. PMID: 26334999
- Heat shock protein 90 is required for ex vivo neutrophil-driven autoantibody-induced tissue damage in experimental epidermolysis bullosa acquisita. PMID: 25739426
顯示更多
收起更多
-
亞細胞定位:Nucleus. Cytoplasm. Melanosome. Cell membrane. Mitochondrion. Note=Identified by mass spectrometry in melanosome fractions from stage I to stage IV.
-
蛋白家族:Heat shock protein 90 family
-
數據庫鏈接:
Most popular with customers
-
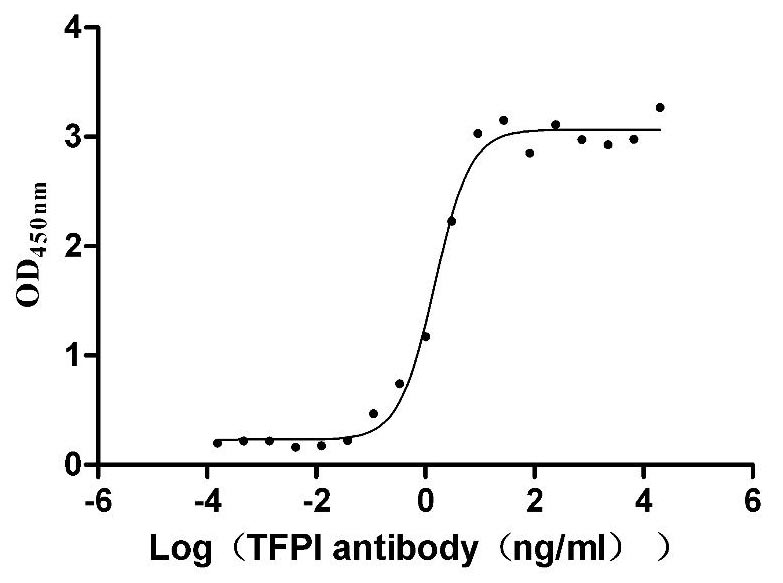
Recombinant Human Tissue factor pathway inhibitor (TFPI), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Rat Intestinal-type alkaline phosphatase 1 (Alpi) (Active)
Express system: Mammalian cell
Species: Rattus norvegicus (Rat)
-
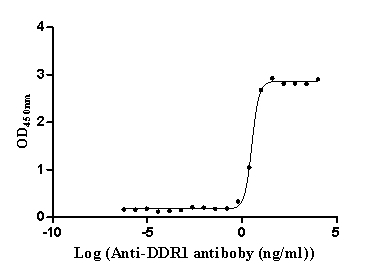
Recombinant Human Epithelial discoidin domain-containing receptor 1 (DDR1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
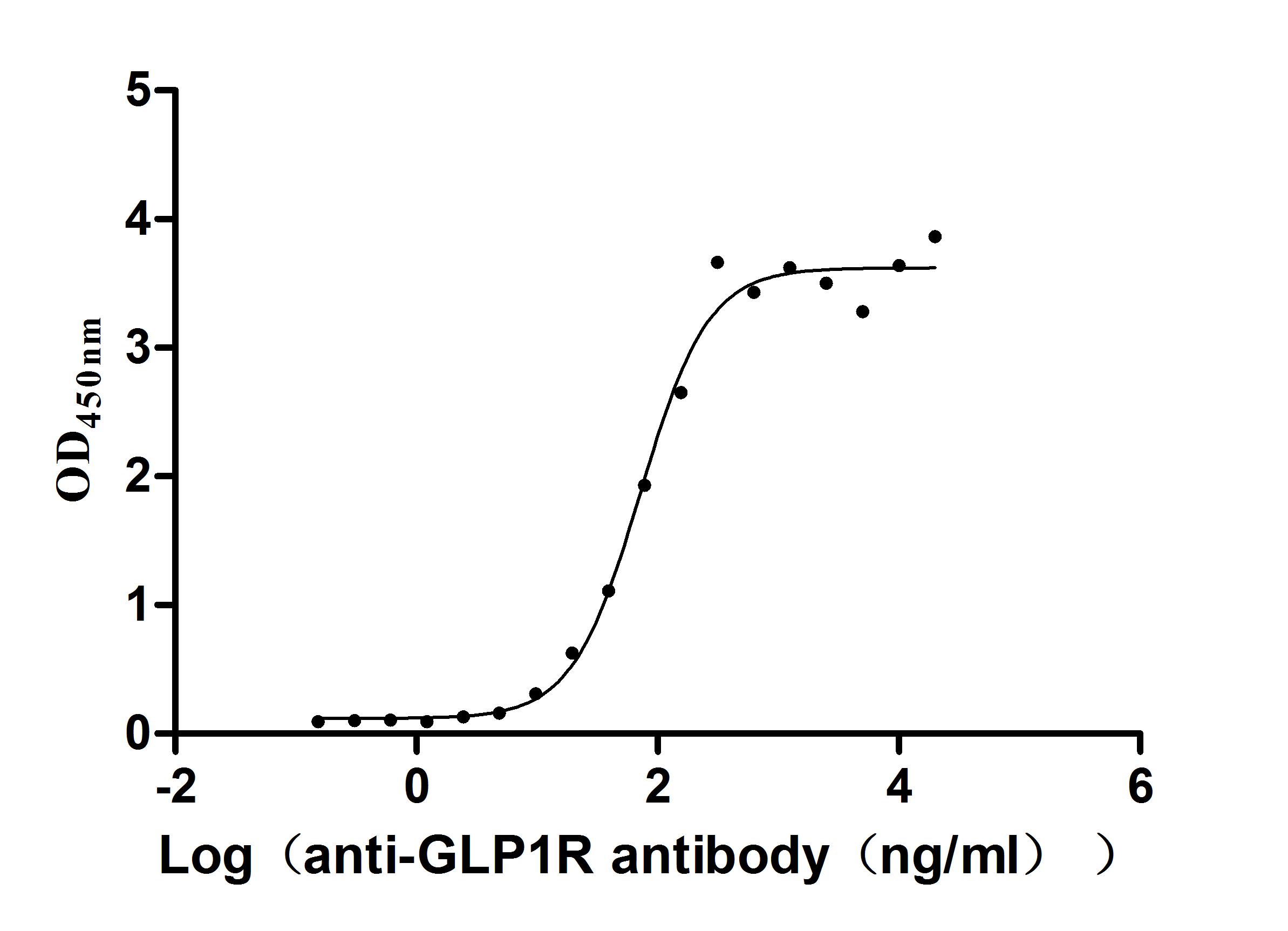
Recombinant Human Glucagon-like peptide 1 receptor (GLP1R), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
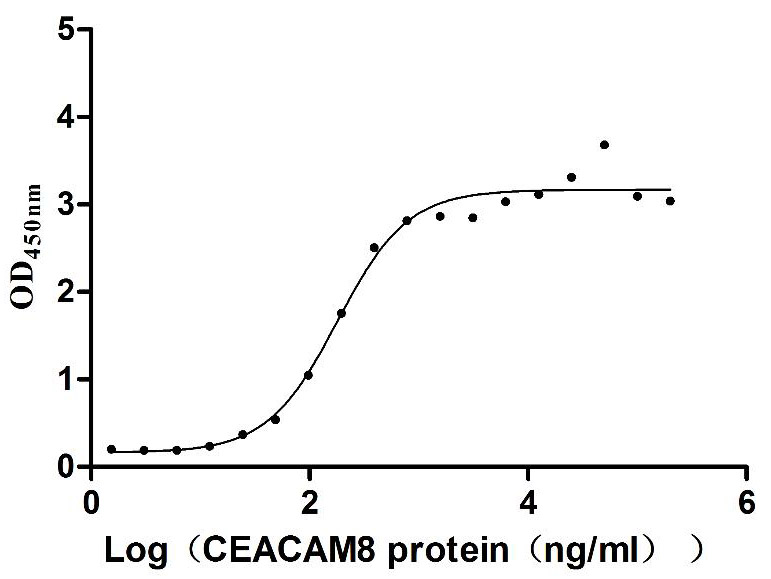
Recombinant Human Carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Desmoglein-2 (DSG2), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Rat Gastric inhibitory polypeptide receptor (Gipr), partial (Active)
Express system: Mammalian cell
Species: Rattus norvegicus (Rat)
-
Recombinant Mouse Cytotoxic and regulatory T-cell molecule (Crtam), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)