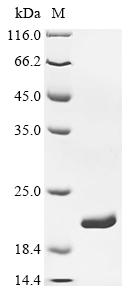
Recombinant Escherichia coli Dihydrofolate reductase (folA)
In Stock-
中文名稱:大腸桿菌folA重組蛋白
-
貨號(hào):CSB-EP006847ENV
-
規(guī)格:¥1836
-
圖片:
-
其他:
產(chǎn)品詳情
-
純度:Greater than 90% as determined by SDS-PAGE.
-
基因名:folA
-
Uniprot No.:
-
種屬:Escherichia coli (strain K12)
-
蛋白長(zhǎng)度:Full Length
-
來源:E.coli
-
分子量:18.8 kDa
-
表達(dá)區(qū)域:1-159aa
-
氨基酸序列MISLIAALAVDRVIGMENAMPWNLPADLAWFKRNTLNKPVIMGRHTWESIGRPLPGRKNIILSSQPGTDDRVTWVKSVDEAIAACGDVPEIMVIGGGRVYEQFLPKAQKLYLTHIDAEVEGDTHFPDYEPDDWESVFSEFHDADAQNSHSYCFEILERR
Note: The complete sequence may include tag sequence, target protein sequence, linker sequence and extra sequence that is translated with the protein sequence for the purpose(s) of secretion, stability, solubility, etc.
If the exact amino acid sequence of this recombinant protein is critical to your application, please explicitly request the full and complete sequence of this protein before ordering. -
蛋白標(biāo)簽:C-terminal 6xHis-tagged
-
產(chǎn)品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
緩沖液:If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol. If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% Trehalose.
-
復(fù)溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
儲(chǔ)存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質(zhì)期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:3-7 business days
-
注意事項(xiàng):Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相關(guān)產(chǎn)品
靶點(diǎn)詳情
-
功能:Key enzyme in folate metabolism. Catalyzes an essential reaction for de novo glycine and purine synthesis, and for DNA precursor synthesis.
-
基因功能參考文獻(xiàn):
- NADP+ not only binds to the native form but also a partially unfolded form of dihydrofolate reductase. PMID: 25367157
- Quantum mechanics/molecular dynamics simulations reveal that the M20 loop conformational dynamics of dihydrofolate reductase (DHFR) is severely restricted at the transition state of the hydride transfer as a result of the M42W/G121V double mutation. PMID: 23297871
- Side-chain conformational heterogeneity of intermediates in the Escherichia coli dihydrofolate reductase catalytic cycle PMID: 23614825
- The data presented here provide a glimpse into the evolutionary trajectory of functional DHFR through its protein sequence space that lead to the diverged binding and catalytic properties of the E. coli and human enzymes. PMID: 23733948
- Present a general kinetic framework that can be used to study conformation changes, apply this framework to E. coli DHFR and find the conformational change occurs predominantly prior to unbinding. PMID: 22641560
- Protein interface remodeling in a chemically induced protein dimer. PMID: 22733548
- Dihydrofolate reductase is bound to endogenous tetrahydrofolate PMID: 22024482
- [review] A general mechanism is presented for folA catalysis that includes multiple intermediates and a complex, multidimensional standard free energy surface. PMID: 22029278
- Only a single peptide from DHFR is found to be substantially more flexible than the Bacillus stearothermophilus-DHFR at 25 degrees C in a region located within the protein interior at the intersection of the cofactor and substrate-binding sites. PMID: 21859100
- Taken together with previous studies in the millisecond time range, a hierarchical assembly of DHFR--in which each subdomain independently folds, subsequently docks, and then anneals into the native conformation after an initial global collapse--emerges. PMID: 21554889
- Thermodynamics and solvent effects on substrate and cofactor binding in Escherichia coli chromosomal dihydrofolate reductase PMID: 21462996
- mutant DHFR that abrogates millisecond-time-scale fluctuation in active site without perturbing structural and electrostatic preorganization; found link between conformational fluctuations on millisecond time scale and chemical step of enzymatic reaction PMID: 21474759
- Fcused on residues 52, 67, 121, and 145 in the four distinct loops of DHFR. All the single-residue deletion mutants showed marked reduction in stability, except for Delta52 in an alphaC-betaC loop. PMID: 20045086
- resulting triple mutants, DM-N18C, DM-R52C, DM-D87C and DM-D132C dihydrofolate reductase, were alkylated with glucose, N-acetylglucosamine, lactose and maltotriose iodoacetamides. PMID: 20412060
- Data show that the M42W mutation alters the dynamics of DHFR and are consistent with theoretical analysis that suggests this mutation disrupts motion that promotes catalysis. PMID: 20073522
- results suggest that dynamics in dihydrofolate reductase are exquisitely "tuned" for every intermediate in the catalytic cycle; structural fluctuations efficiently channel the enzyme through functionally relevant conformational space. PMID: 20080605
- These results suggest that through electrostatic interactions Arg44 plays a functional role in retaining the cofactor binding affinity at the cost of the Escherichia coli dihydrofolate reductase stability. PMID: 20043879
- Lys-32 residues have a role in the ionic interaction in R67 dihydrofolate reductase PMID: 15333636
- the hydroxyl group of Tyr-69 of DFHR is important for interactions with NADPH, whereas both the hydroxyl group and hydrophobic ring atoms of the Tyr-69 residues are necessary for proper interactions with dihydrofolate PMID: 15333637
- structural and functional alterations induced by peroxynitrite may play a direct role in compromising DHFR function in multiple pathological conditions PMID: 15639221
- biophysical analysis of immobilized and native Escherichia coli dihydrofolate reductase PMID: 16258053
- Results show that mutant dihydrofolate reductase has reduced catalytic activity. PMID: 16363797
- characterization of higher energy conformational substates of dihydrofolate reductase using using nuclear magnetic resonance relaxation dispersion PMID: 16973882
- DHFR structure from neutron diffraction studies provides insights into dynamics, active-site protonation states, and solvation pattern of the E. coli enzyme. PMID: 17130456
- The folding trajectory of this alpha/beta-type protein (DHFR) is located between those of alpha-helical and beta-sheet proteins, suggesting that native structure determines the folding landscape. PMID: 17331539
- Several mutations were found to grant resistance to trimethoprim, both by reducing the binding affinity of the enzyme for the drug, and by increasing the activity of the enzyme. PMID: 17451440
顯示更多
收起更多
-
蛋白家族:Dihydrofolate reductase family
-
數(shù)據(jù)庫鏈接:
KEGG: ecj:JW0047
STRING: 316385.ECDH10B_0049
Most popular with customers
-
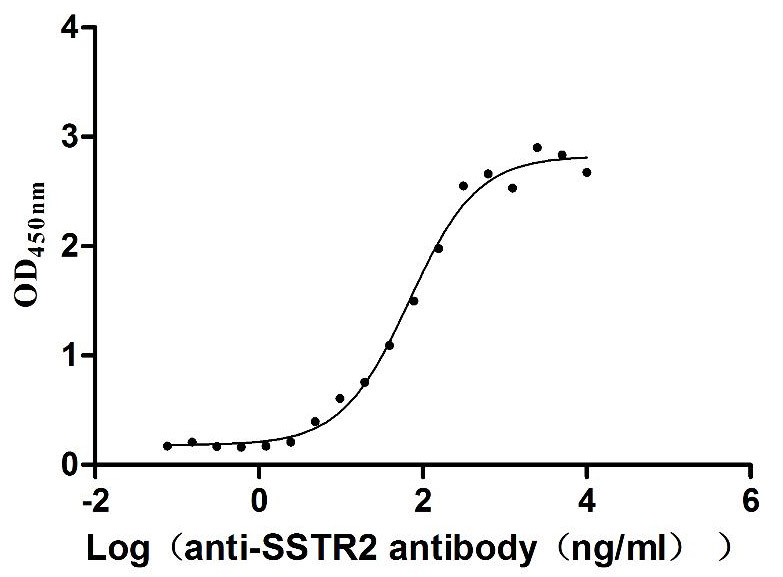
Recombinant Human Somatostatin receptor type 2 (SSTR2)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
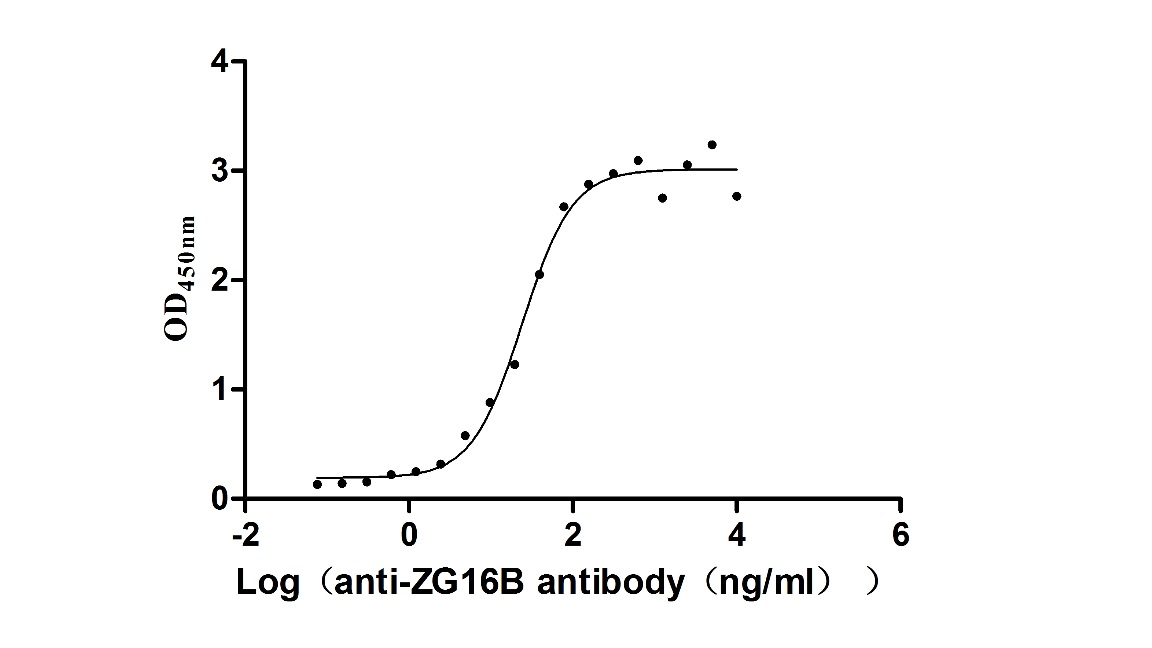
Recombinant Macaca fascicularis zymogen granule protein 16 homolog B (ZG16B) (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
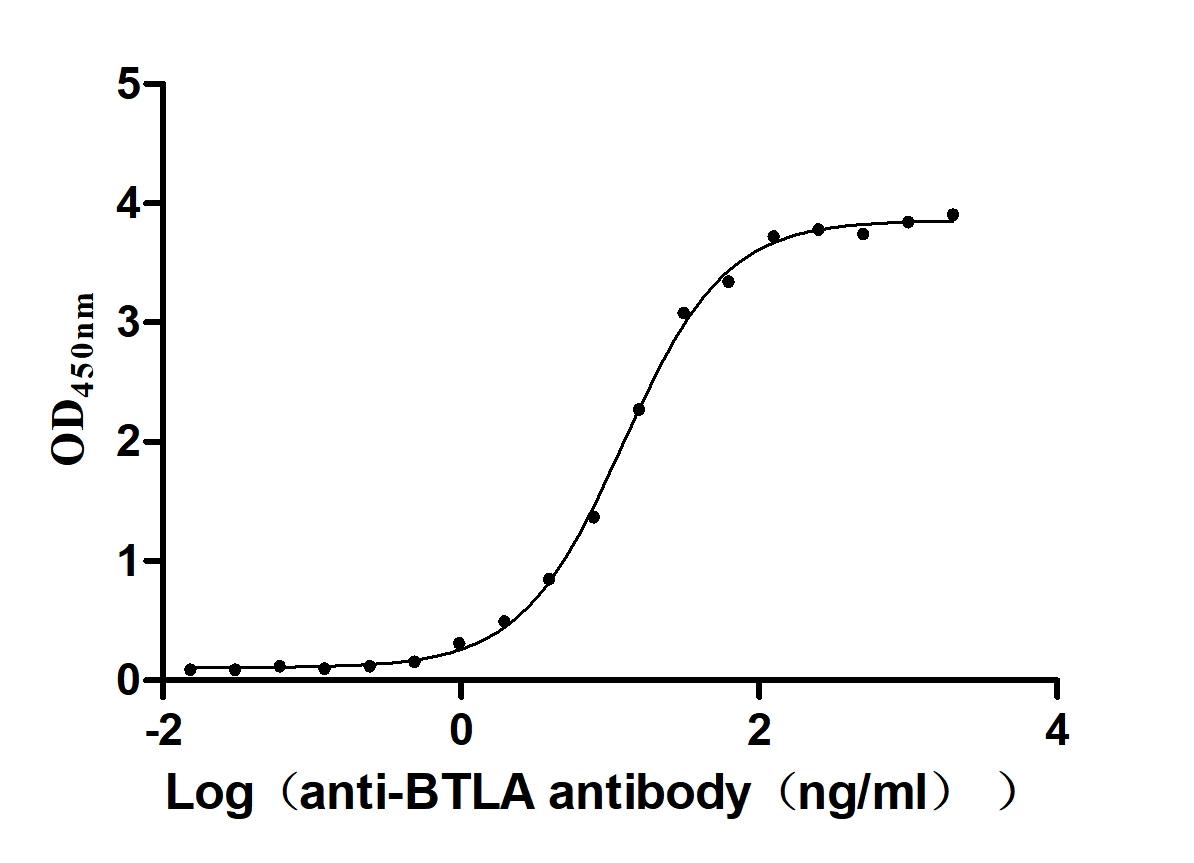
Recombinant Human B- and T-lymphocyte attenuator(BTLA), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
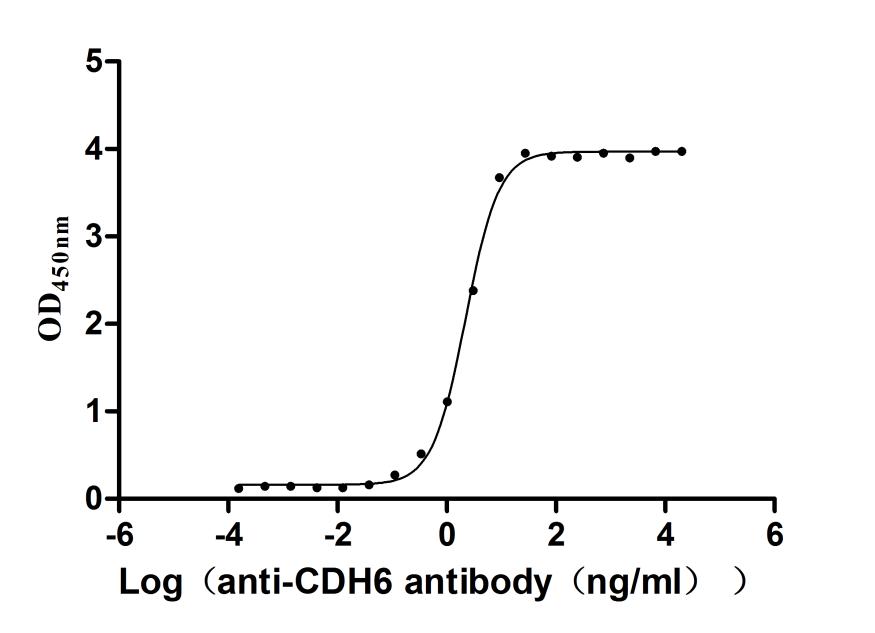
Recombinant Macaca fascicularis Cadherin 6(CDH6),partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
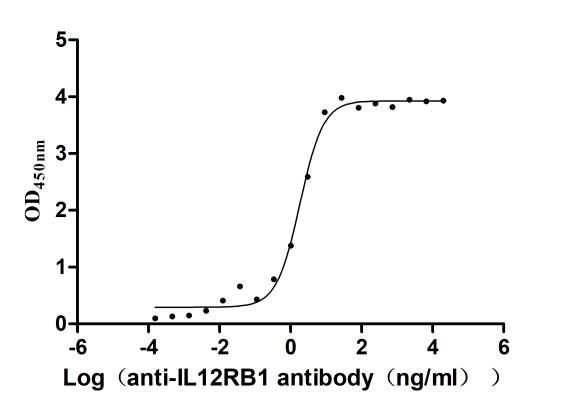
Recombinant Human Interleukin-12 receptor subunit beta-1(IL12RB1),partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Express system: Mammalian cell
Species: Homo sapiens (Human)