-
中文名稱:folA兔多克隆抗體
-
貨號:CSB-PA006847XA01ENV
-
規(guī)格:¥440
-
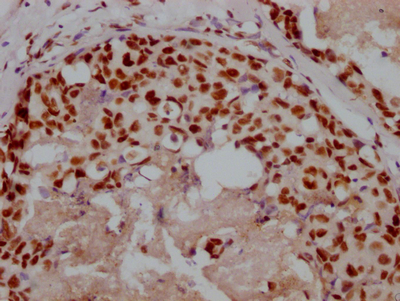
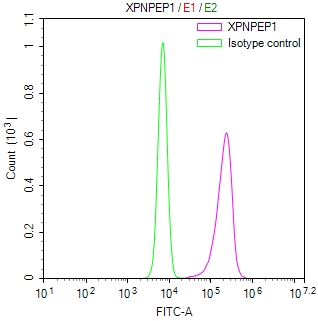
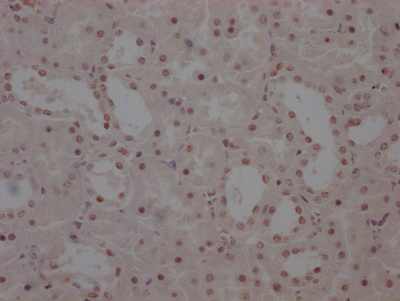
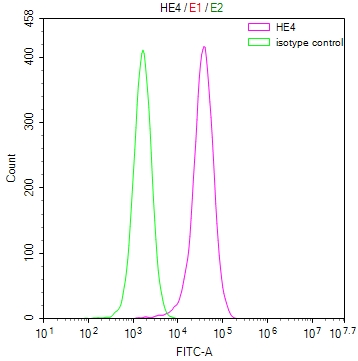
圖片:
-
其他:
產(chǎn)品詳情
-
Uniprot No.:
-
基因名:folA
-
別名:Dihydrofolate reductase (EC 1.5.1.3) folA tmrA b0048 JW0047
-
反應(yīng)種屬:Escherichia coli (strain K12)
-
免疫原:Recombinant Escherichia coli (strain K12) folA protein (1-159aa)
-
免疫原種屬:Escherichia coli (strain K12)
-
標(biāo)記方式:Non-conjugated
-
克隆類型:Polyclonal
-
抗體亞型:IgG
-
純化方式:Affinity-chromatography
-
濃度:It differs from different batches. Please contact us to confirm it.
-
保存緩沖液:Preservative: 0.03% Proclin 300
Constituents: 50% Glycerol, 0.01M PBS, pH 7.4 -
產(chǎn)品提供形式:Liquid
-
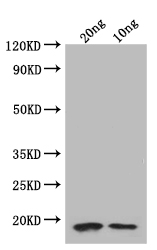
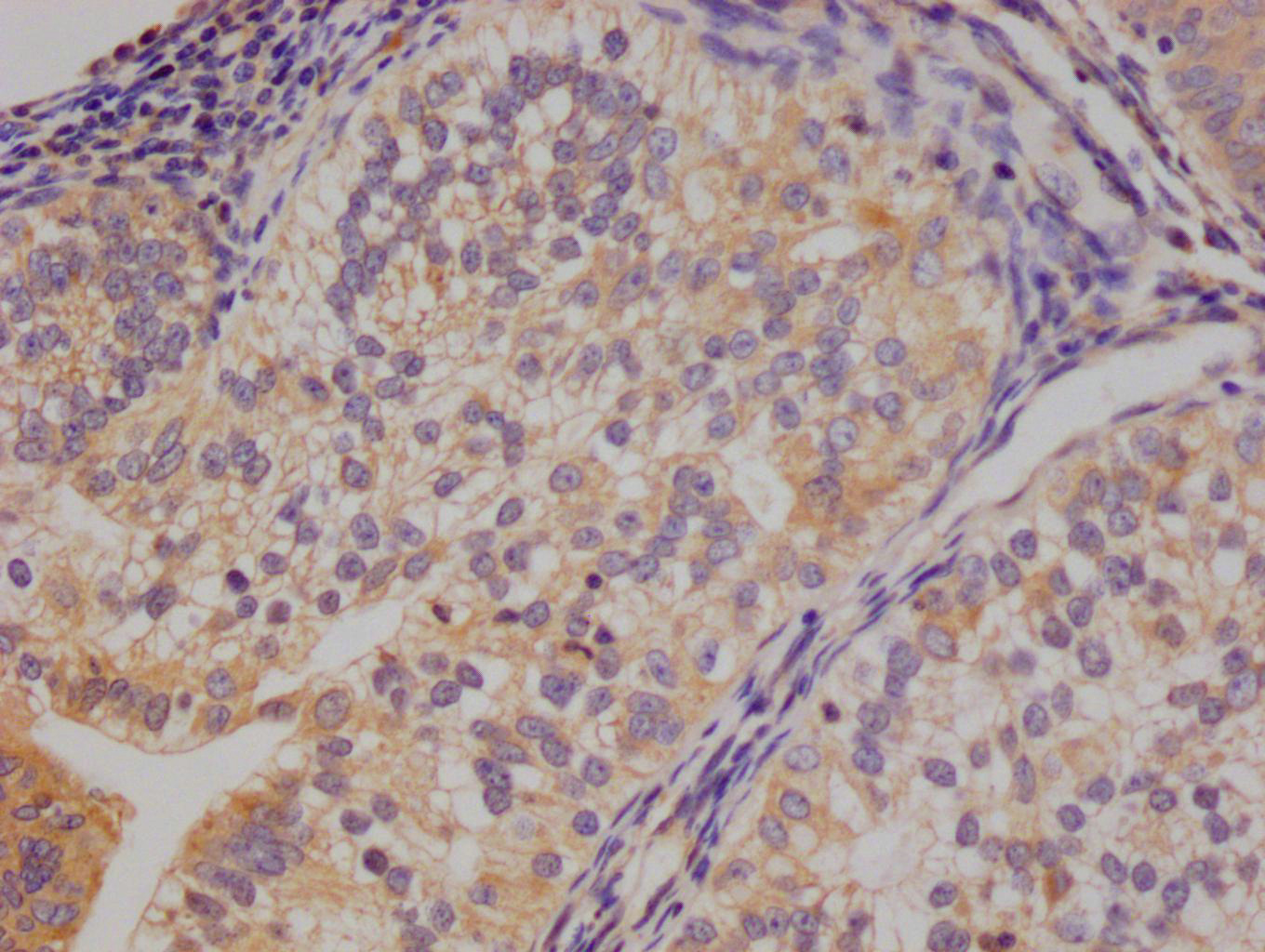
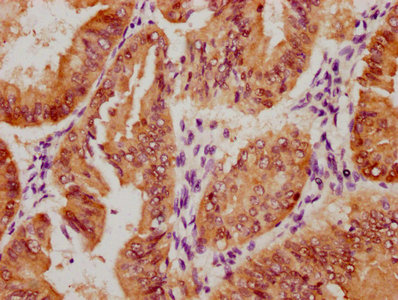
應(yīng)用范圍:ELISA, WB
-
Protocols:
-
儲存條件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
貨期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
-
用途:For Research Use Only. Not for use in diagnostic or therapeutic procedures.
相關(guān)產(chǎn)品
靶點詳情
-
功能:Key enzyme in folate metabolism. Catalyzes an essential reaction for de novo glycine and purine synthesis, and for DNA precursor synthesis.
-
基因功能參考文獻(xiàn):
- NADP+ not only binds to the native form but also a partially unfolded form of dihydrofolate reductase. PMID: 25367157
- Quantum mechanics/molecular dynamics simulations reveal that the M20 loop conformational dynamics of dihydrofolate reductase (DHFR) is severely restricted at the transition state of the hydride transfer as a result of the M42W/G121V double mutation. PMID: 23297871
- Side-chain conformational heterogeneity of intermediates in the Escherichia coli dihydrofolate reductase catalytic cycle PMID: 23614825
- The data presented here provide a glimpse into the evolutionary trajectory of functional DHFR through its protein sequence space that lead to the diverged binding and catalytic properties of the E. coli and human enzymes. PMID: 23733948
- Present a general kinetic framework that can be used to study conformation changes, apply this framework to E. coli DHFR and find the conformational change occurs predominantly prior to unbinding. PMID: 22641560
- Protein interface remodeling in a chemically induced protein dimer. PMID: 22733548
- Dihydrofolate reductase is bound to endogenous tetrahydrofolate PMID: 22024482
- [review] A general mechanism is presented for folA catalysis that includes multiple intermediates and a complex, multidimensional standard free energy surface. PMID: 22029278
- Only a single peptide from DHFR is found to be substantially more flexible than the Bacillus stearothermophilus-DHFR at 25 degrees C in a region located within the protein interior at the intersection of the cofactor and substrate-binding sites. PMID: 21859100
- Taken together with previous studies in the millisecond time range, a hierarchical assembly of DHFR--in which each subdomain independently folds, subsequently docks, and then anneals into the native conformation after an initial global collapse--emerges. PMID: 21554889
- Thermodynamics and solvent effects on substrate and cofactor binding in Escherichia coli chromosomal dihydrofolate reductase PMID: 21462996
- mutant DHFR that abrogates millisecond-time-scale fluctuation in active site without perturbing structural and electrostatic preorganization; found link between conformational fluctuations on millisecond time scale and chemical step of enzymatic reaction PMID: 21474759
- Fcused on residues 52, 67, 121, and 145 in the four distinct loops of DHFR. All the single-residue deletion mutants showed marked reduction in stability, except for Delta52 in an alphaC-betaC loop. PMID: 20045086
- resulting triple mutants, DM-N18C, DM-R52C, DM-D87C and DM-D132C dihydrofolate reductase, were alkylated with glucose, N-acetylglucosamine, lactose and maltotriose iodoacetamides. PMID: 20412060
- Data show that the M42W mutation alters the dynamics of DHFR and are consistent with theoretical analysis that suggests this mutation disrupts motion that promotes catalysis. PMID: 20073522
- results suggest that dynamics in dihydrofolate reductase are exquisitely "tuned" for every intermediate in the catalytic cycle; structural fluctuations efficiently channel the enzyme through functionally relevant conformational space. PMID: 20080605
- These results suggest that through electrostatic interactions Arg44 plays a functional role in retaining the cofactor binding affinity at the cost of the Escherichia coli dihydrofolate reductase stability. PMID: 20043879
- Lys-32 residues have a role in the ionic interaction in R67 dihydrofolate reductase PMID: 15333636
- the hydroxyl group of Tyr-69 of DFHR is important for interactions with NADPH, whereas both the hydroxyl group and hydrophobic ring atoms of the Tyr-69 residues are necessary for proper interactions with dihydrofolate PMID: 15333637
- structural and functional alterations induced by peroxynitrite may play a direct role in compromising DHFR function in multiple pathological conditions PMID: 15639221
- biophysical analysis of immobilized and native Escherichia coli dihydrofolate reductase PMID: 16258053
- Results show that mutant dihydrofolate reductase has reduced catalytic activity. PMID: 16363797
- characterization of higher energy conformational substates of dihydrofolate reductase using using nuclear magnetic resonance relaxation dispersion PMID: 16973882
- DHFR structure from neutron diffraction studies provides insights into dynamics, active-site protonation states, and solvation pattern of the E. coli enzyme. PMID: 17130456
- The folding trajectory of this alpha/beta-type protein (DHFR) is located between those of alpha-helical and beta-sheet proteins, suggesting that native structure determines the folding landscape. PMID: 17331539
- Several mutations were found to grant resistance to trimethoprim, both by reducing the binding affinity of the enzyme for the drug, and by increasing the activity of the enzyme. PMID: 17451440
顯示更多
收起更多
-
蛋白家族:Dihydrofolate reductase family
-
數(shù)據(jù)庫鏈接:
KEGG: ecj:JW0047
STRING: 316385.ECDH10B_0049
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-