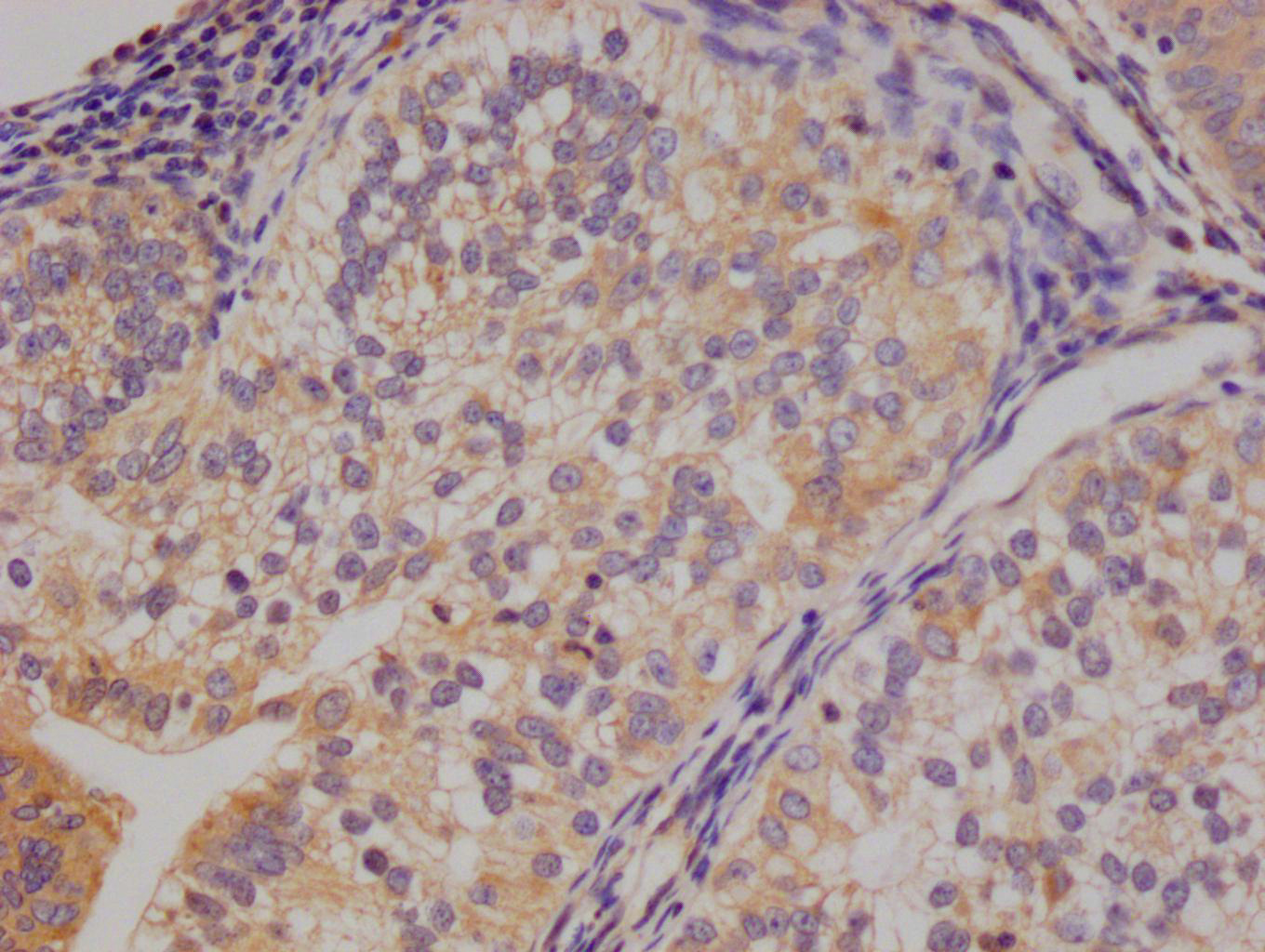
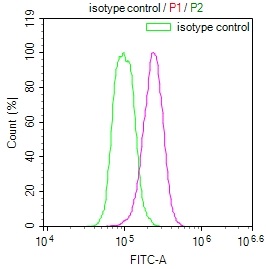
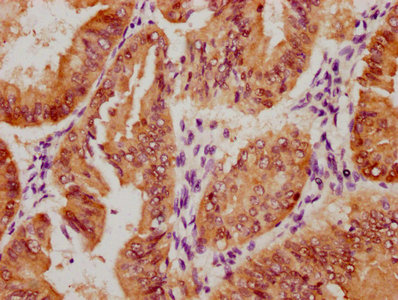
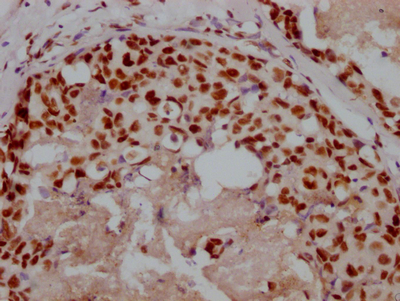
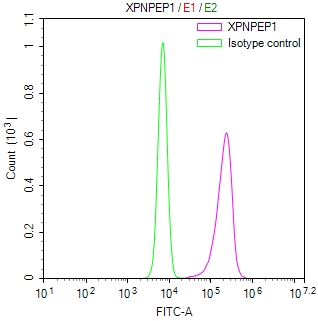
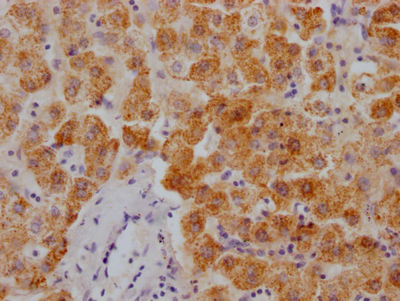
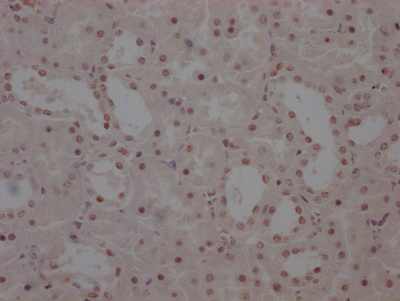
OSBP Antibody
-
中文名稱:OSBP兔多克隆抗體
-
貨號:CSB-PA017243GA01HU
-
規格:¥3,900
-
其他:
產品詳情
-
Uniprot No.:
-
基因名:OSBP
-
別名:OSBP 1 antibody; OSBP antibody; OSBP1_HUMAN antibody; Oxysterol binding protein 1 antibody; Oxysterol-binding protein 1 antibody
-
宿主:Rabbit
-
反應種屬:Human,Mouse,Rat
-
免疫原:Human OSBP
-
免疫原種屬:Homo sapiens (Human)
-
抗體亞型:IgG
-
純化方式:Antigen Affinity Purified
-
濃度:It differs from different batches. Please contact us to confirm it.
-
保存緩沖液:PBS with 0.1% Sodium Azide, 50% Glycerol, pH 7.3. -20°C, Avoid freeze / thaw cycles.
-
產品提供形式:Liquid
-
應用范圍:ELISA,WB,IHC,IP
-
Protocols:
-
儲存條件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
貨期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
-
用途:For Research Use Only. Not for use in diagnostic or therapeutic procedures.
相關產品
靶點詳情
-
功能:Lipid transporter involved in lipid countertransport between the Golgi complex and membranes of the endoplasmic reticulum: specifically exchanges sterol with phosphatidylinositol 4-phosphate (PI4P), delivering sterol to the Golgi in exchange for PI4P, which is degraded by the SAC1/SACM1L phosphatase in the endoplasmic reticulum. Binds cholesterol and a range of oxysterols including 25-hydroxycholesterol. Cholesterol binding promotes the formation of a complex with PP2A and a tyrosine phosphatase which dephosphorylates ERK1/2, whereas 25-hydroxycholesterol causes its disassembly. Regulates cholesterol efflux by decreasing ABCA1 stability.
-
基因功能參考文獻:
- the component proteins of the machinery, OSBP, VAP, SAC1, and PITPNB, are all essential host factors for AiV replication. Importantly, the machinery is directly recruited to the RNA replication sites through previously unknown interactions of VAP/OSBP/SAC1 with the AiV proteins and with ACBD3. PMID: 29367253
- results demonstrate that Sac1 expression in either the ER or Golgi apparatus has a minimal impact on the PI-4P that regulates OSBP activity or recruitment to contact sites PMID: 28471037
- Cholesterol transfer, PI4P consumption, and control of membrane lipid order by endogenous OSBP have been described. PMID: 28978670
- Data suggest that OSBP shifts the distribution of phosphatidylinositol 4-phosphate upon localization to endoplasmic reticulum-Golgi contact sites. PMID: 26601944
- Our results identify OspB as a regulator of mTORC1 and mTORC1-dependent cell proliferation early during S. flexneri infection and establish a role for IQGAP1 in mTORC1 signaling PMID: 26473364
- These results suggest that poliovirus proteins modulate PI4KB activity and provide PI4P for recruitment of OSBP to accumulate unesterified cholesterol on virus-induced membrane structure for formation of a virus replication complex. PMID: 24527995
- OSBP-mediated back transfer of phosphatidylinositol 4-phosphate might coordinate the transfer of other lipid species at the endoplasmic reticulum-Golgi interface. PMID: 24209621
- OSBP is required for efficient replication of intracellular S. Typhimurium. PMID: 21988961
- Data indicate that phosphorylation on two serine-rich motifs, S381-S391 (site 1) and S192, S195, S200 (site 2), specifically controls oxysterol-binding protein (OSBP) activity at the endoplasmic reticulum (ER). PMID: 22875984
- PKD negatively regulates HCV secretion/release by attenuating OSBP and CERT functions by phosphorylation inhibition. This study identifies the key role of the Golgi components in the HCV maturation process. PMID: 21285358
- Results identify a novel substrate of protein kinase D at the Golgi, the oxysterol-binding protein OSBP. PMID: 20444975
- This review summarizes recent evidence of sterol transfer activity by OSBP, suggesting seemingly disparate functions that could be the result of alterations in membrane sterol distribution or ancillary to this primary activity. PMID: 20545625
- Electrostatic interaction between oxysterol-binding protein and VAMP-associated protein A revealed by NMR and mutagenesis studies. PMID: 20178991
- OSBP was found to function as a cholesterol-binding scaffolding protein coordinating the activity of two phosphatases to control the extracellular signal-regulated kinase (ERK) signaling pathway PMID: 15746430
- Regulation of ceramide transport protein by OSBP, sterols, and vesicle-associated protein reveals a novel mechanism for integrating sterol regulatory signals with ceramide transport and phingomyelin synthesis in the Golgi apparatus. PMID: 16571669
- OSBP is able to sense both membrane cholesterol and oxidized sterols and link this information to the ERK1/2 signaling pathway. PMID: 18165705
- functional role of OSBP in the HCV maturation process. PMID: 19570870
顯示更多
收起更多
-
亞細胞定位:Cytoplasm, cytosol. Cytoplasm, perinuclear region. Golgi apparatus membrane; Peripheral membrane protein. Endoplasmic reticulum membrane; Peripheral membrane protein. Golgi apparatus, trans-Golgi network.
-
蛋白家族:OSBP family
-
組織特異性:Widely expressed.
-
數據庫鏈接:
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-