Recombinant Saccharomyces cerevisiae Heat shock protein SSB1 (SSB1), partial
-
中文名稱:釀酒酵母SSB1重組蛋白
-
貨號:CSB-YP320875SVG
-
規(guī)格:
-
來源:Yeast
-
其他:
-
中文名稱:釀酒酵母SSB1重組蛋白
-
貨號:CSB-EP320875SVG
-
規(guī)格:
-
來源:E.coli
-
其他:
-
中文名稱:釀酒酵母SSB1重組蛋白
-
貨號:CSB-EP320875SVG-B
-
規(guī)格:
-
來源:E.coli
-
共軛:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名稱:釀酒酵母SSB1重組蛋白
-
貨號:CSB-BP320875SVG
-
規(guī)格:
-
來源:Baculovirus
-
其他:
-
中文名稱:釀酒酵母SSB1重組蛋白
-
貨號:CSB-MP320875SVG
-
規(guī)格:
-
來源:Mammalian cell
-
其他:
產(chǎn)品詳情
-
純度:>85% (SDS-PAGE)
-
基因名:SSB1
-
Uniprot No.:
-
別名:SSB1; YG101; YDL229W; Ribosome-associated molecular chaperone SSB1; EC 3.6.4.10; Cold-inducible protein YG101; Heat shock protein SSB1; Hsp70 chaperone Ssb
-
種屬:Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast)
-
蛋白長度:Partial
-
蛋白標(biāo)簽:Tag?type?will?be?determined?during?the?manufacturing?process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
產(chǎn)品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
復(fù)溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
儲存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質(zhì)期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事項:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
靶點詳情
-
功能:Ribosome-bound, Hsp70-type chaperone that assists in the cotranslational folding of newly synthesized proteins in the cytosol. Stimulates folding by interacting with nascent chains, binding to short, largely hydrophobic sequences exposed by unfolded proteins, thereby stabilizing longer, more slowly translated, and aggregation-prone nascent polypeptides and domains that cannot fold stably until fully synthesized. The Hsp70-protein substrate interaction depends on ATP-binding and on allosteric regulation between the NBD and the SBD. The ATP-bound state is characterized by a fast exchange rate of substrate (low affinity state), while in the ADP-bound state exchange is much slower (high affinity state). During the Hsp70 cycle, the chaperone switches between the ATP-bound state (open conformation) and the ADP-bound state (closed conformation) by major conformational rearrangements involving mainly the lid domain. Ssb cooperates with a specific Hsp40/Hsp70 co-chaperone termed the ribosome-associated complex (RAC), which stimulates the ATPase activity of the ribosome-associated pool of Ssbs and switches it to the high affinity substrate binding state. Hsp110 chaperone SSE1 and FES1 act as nucleotide exchange factors that cause substrate release.
-
基因功能參考文獻(xiàn):
- A positively charged region in the alpha-helical lid domain of SSB is identified. It is strictly required for ribosome binding. Crosslinking shows that Ssb binds close to the tunnel exit via contacts with both, ribosomal proteins and rRNA, and that specific contacts can be correlated with switching between the open (ATP-bound) and closed (ADP-bound) conformation. PMID: 27882919
- Data show that the absence of either Ssb1/2 or Sch9 causes enhanced ribosome aggregation. PMID: 29038496
- Study reveals molecular features of chaperone action during translation in eukaryotes by providing proteome-wide Ssb (two isoforms Ssb1 and Ssb2) interaction profiles with nascent chains at near-codon resolution. The Ssb interactome is broader than previously thought and includes nascent mitochondrial and endoplasmic reticulum- (ER) translocated proteins. PMID: 28708998
- the ribosome-associated Hsp70 Ssb is redistributed away from Sup35 prion aggregates to the nascent chains, leading to an array of aggregation phenotypes that can mimic both overexpression and deletion of Ssb. PMID: 27828954
- Study finds that SSB (i.e. closely related isoforms Ssb1 and Ssb2) binds to a subset of nascent polypeptides whose intrinsic properties and slow translation rates hinder efficient cotranslational folding. PMID: 23332755
- The ribosome-bound chaperone system consisting of the ribosome-associated complex (RAC) and the Hsp70 homologs SSB1 and SSB2 are required to stabilize translationally repressed ribosome-polylysine protein complexes. PMID: 23007158
- Results suggest that RAC and Ssb1/2p are crucial in maintaining translational fidelity beyond their postulated role as chaperones for nascent polypeptides. PMID: 15456889
- ssb and zuo1 have a role in cation influx in Saccharomyces cerevisiae membranes PMID: 15643063
- A plausible role of the Ssb1 chaperone is to stabilize genetic networks, thus making them more tolerant to malfunctioning of their constituents. PMID: 16849597
- Zuo1 and Ssz1 together with the Hsp70 homolog Ssb1/2 form a functional triad involved in translation and early polypeptide folding processes. PMID: 17901048
顯示更多
收起更多
-
亞細(xì)胞定位:Cytoplasm.
-
蛋白家族:Heat shock protein 70 family, Ssb-type Hsp70 subfamily
-
數(shù)據(jù)庫鏈接:
KEGG: sce:YDL229W
STRING: 4932.YDL229W
Most popular with customers
-
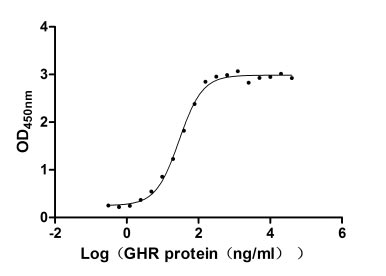
Recombinant Human Growth hormone receptor (GHR), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
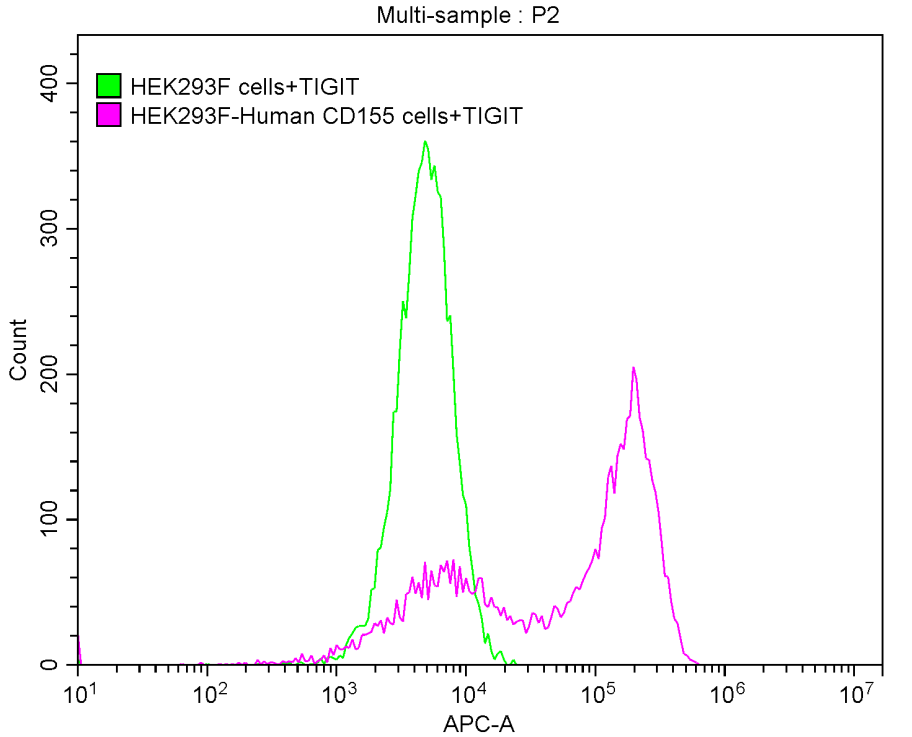
Recombinant Human T-cell immunoreceptor with Ig and ITIM domains (TIGIT), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Poliovirus receptor (PVR) (I340M), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Interleukin-17A (IL17A) (T26A) (Active)
Express system: Baculovirus
Species: Homo sapiens (Human)
-
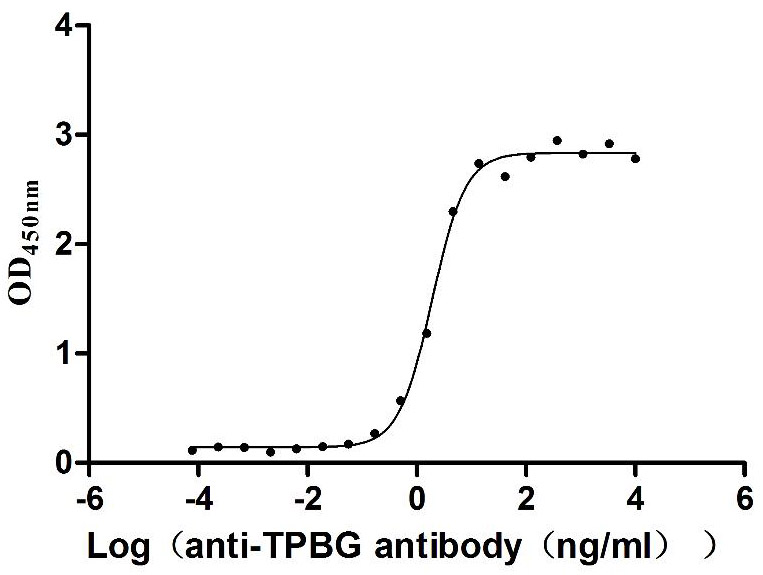
Recombinant Macaca fascicularis Trophoblast glycoprotein (TPBG), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
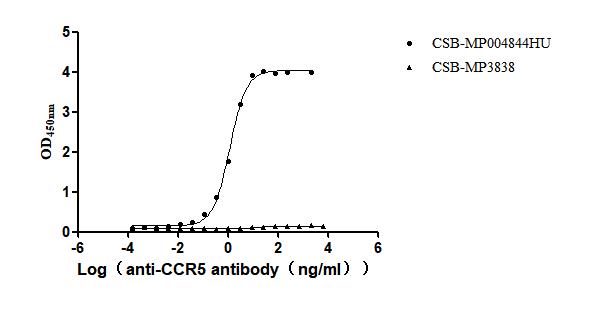
Recombinant Human C-C chemokine receptor type 5 (CCR5)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)


-AC1.jpg)
-AC1.jpg)