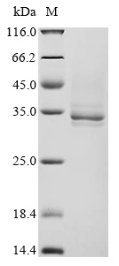
Recombinant Escherichia coli Outer membrane protein assembly factor BamA (BamA), partial
In Stock-
貨號:CSB-YP364271ENV1
-
規格:¥2616
-
圖片:
-
其他:
產品詳情
-
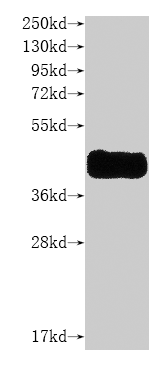
純度:Greater than 85% as determined by SDS-PAGE.
-
基因名:bamA
-
Uniprot No.:
-
別名:(Omp85)
-
種屬:Escherichia coli (strain K12)
-
蛋白長度:Partial
-
來源:Yeast
-
分子量:30 kDa
-
表達區域:175-424aa
-
氨基酸序列AEIQQINIVGNHAFTTDELISHFQLRDEVPWWNVVGDRKYQKQKLAGDLETLRSYYLDRGYARFNIDSTQVSLTPDKKGIYVTVNITEGDQYKLSGVEVSGNLAGHSAEIEQLTKIEPGELYNGTKVTKMEDDIKKLLGRYGYAYPRVQSMPEINDADKTVKLRVNVDAGNRFYVRKIRFEGNDTSKDAVLRREMRQMEGAWLGSDLVDQGKERLNRLGFFETVDTDTQRVPGSPDQVDVVYKVKERNTG
Note: The complete sequence may include tag sequence, target protein sequence, linker sequence and extra sequence that is translated with the protein sequence for the purpose(s) of secretion, stability, solubility, etc.
If the exact amino acid sequence of this recombinant protein is critical to your application, please explicitly request the full and complete sequence of this protein before ordering. -
蛋白標簽:C-terminal 6xHis-tagged
-
產品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
緩沖液:If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol. If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% Trehalose.
-
復溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20°C/-80°C. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
儲存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:3-7 business days
-
注意事項:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相關產品
靶點詳情
-
功能:Part of the outer membrane protein assembly complex (Bam), which is involved in assembly and insertion of beta-barrel proteins into the outer membrane. Constitutes, with BamD, the core component of the assembly machinery. Efficient substrate folding and insertion into the outer membrane requires all 5 subunits. A lateral gate may open between the first and last strands of the BamA beta-barrel that allows substrate to insert into the outer membrane; comparison of the structures of complete and nearly complete Bam complexes show there is considerable movement of all 5 proteins.; (Microbial infection) Acts as a receptor for CdiA-EC93, the contact-dependent growth inhibition (CDI) effector of E.coli strain EC93; antibodies against extracellular epitopes decrease CDI. Its role in CDI is independent of the other Bam complex components. Is not the receptor for CdiA from E.coli strain 536 / UPEC, which does not have the same mode of toxicity as CdiA from strain EC93; the decreased expression of bamA101 in some experiments decreases the level of outer membrane proteins in general. Susceptibility to CdiA-EC93 is dependent on E.coli BamA; replacing BamA with the gene from S.typhimurium LT2, E.cloacae ATCC 13047 or D.dadantii 3937 renders cells resistant to CdiA-EC93. Cells with BamA from another bacteria no longer form CdiA-EC93-induced aggregates with EC93 cells. A chimera in which E.cloacae extracellular loops 6 and 7 are replaced with loops 6 and 7 from E.coli is susceptible to CdiA-EC93 and to CdiA-CT from strain 536 / UPEC.
-
基因功能參考文獻:
- Free-energy calculations of lateral gate opening revealed a significantly lower barrier to opening in the C-terminal kinked conformation; mutagenesis experiments confirm the relevance of C-terminal kinking to BamA structure and function. PMID: 30087180
- Studied how the Bam complex accelerates folding and insertion through the assembly of a slow-folding beta-barrel substrate, LptD. Results suggest a mechanism in which substrate recruitment by periplasmic BamD regulates extracellular loop interactions to activate BamA for folding and insertion. PMID: 29463713
- we studied the assembly of an essential beta-barrel substrate for the Bam complex, BamA. By mutating conserved residues in the beta-barrel domain of this protein, we generated three assembly-defective BamA substrates that stall early in the folding process in the periplasm. Two of the three defective substrates, which harbor mutations within beta-strands, fail to associate productively with the Bam complex PMID: 28223520
- Data show that LptD is folded around LptE, and both components interact with the two essential beta-barrel assembly machine (Bam) components, BamA and BamD. PMID: 27439868
- The authors show that electrostatic interactions between the two essential proteins BamA and BamD coordinate conformational changes upon binding of unfolded substrate that allow the assembly reaction to proceed. PMID: 28760846
- Study investigated BamA flexibility using molecular dynamics simulations of BamA embedded in a model Escherichia coli outer membrane, reported that POTRA-membrane interactions influence the set of observed conformations PMID: 27332128
- BamD and BamA mutations cause a marked decrease in the levels of multimeric proteins. PMID: 27161117
- BamA alone can repeatedly facilitate the folding of OMPs PMID: 26394056
- Data show that disulfide crosslinks prevented lateral opening and exit pore formation resulting in a loss of outer membrane proteins BamA function, which can be fully rescued by the reductant tris(2-carboxyethyl)phosphine. PMID: 24980798
- structural analysis of BamB bound to a periplasmic domain fragment of BamA, the central component of the beta-barrel assembly machine PMID: 25468906
- BamA has five polypeptide transport-associated domains in its N-terminus, all of which are essential for BamA protein function. PMID: 24376817
- study determined the crystal structure of the C-terminal transmembrane domain of BamA at 2.6 A resolution; findings suggest the dome over the barrel may play an important role in maintaining the efficiency of OMP biogenesis PMID: 24619089
- These results suggest that the periplasmic region of BamA is firmly attached to the beta-barrel and does not experience fast global motion around the angle between POTRA 2 and 3. PMID: 24530687
- Our direct coupling analysis of BamA implicates residues R661 and D740 in a functional interaction. We find that the substitutions R661G and D740G each confer OM permeability defects and destabilize the BamA b-barrel PMID: 23934888
- Pull-down and Western blotting assays indicate that BamB interacts directly with the POTRA 1-3 domain of BamA PMID: 22948914
- the conserved (641)RGF(643) residues of the BamA L6 loop are important for BamA folding and biogenesis PMID: 22753067
- results imply that BamA and BamD interact directly with outer membrane protein substrates PMID: 22331884
- Taken together, these findings suggest that BamE modulates the conformation of BamA, likely through its interactions with BamD. PMID: 22178970
- Conditions that increase the folding of BamA demonstrate the ability of the reconstituted complex to catalyze more than one round of substrate assembly. PMID: 21823654
- the crystal structure of BamA POTRA4-5 has been determined to 1.50 A resolution with an R factor of 14.7% and an Rfree of 18.9% PMID: 21795783
- The data suggest that SurA and BamA POTRA 1 domain function in concert to assist folding and assembly of most beta-barrel outer membrane proteins except for TolC. PMID: 20598079
- The authors demonstrate that (i) BamA is essential for biogenesis of the trimeric auotransporter adhesins YadA, (ii) BamA interacts directly with YadA, (iii) the C-terminal amino acid motif of YadA is important for the BamA-dependent assembly. PMID: 20815824
- the periplasmic chaperone SurA and subunits of the Bam (Omp85) complex catalyse the insertion and assembly of outer-membrane proteins PMID: 19815580
- YaeT may have a role in outer membrane protein assembly in E. coli PMID: 15951436
- YaeT acts as a general outer membrane proteins assembly factor. PMID: 16102012
- YaeT, through its interaction with other outer membrane lipoproteins, forms a functional complex to assemble Escherichia coli membrane protein. PMID: 16824102
- YaeT is composed of two distinct domains, an amino-terminal periplasmic and a carboxy-terminal membrane domain; the periplasmic domain proves to be essential for in vivo function of YaeT PMID: 16829683
- Here we show that an essential and highly conserved gene product, YaeT, is the surface molecule recognized by the majority (ca. 70%) of Stx phages via conserved tail spike proteins PMID: 17693515
- crystal structure of a periplasmic fragment of YaeT reveals the polypeptide transport-associated (POTRA) domain fold and suggests a model for how POTRA domains can bind different peptide sequences PMID: 17702946
- Data show that YaeT-YfgL interaction invloves the region encompassing L173, L175, and R176 of YfgL and it was found that altering residues D227 and D229 in another region of YfgL from E221 to D229 resulted in defective YaeT bindings. PMID: 18165306
- Characterization of mutants resistant to contact-dependent growth inhibition (CDI) allowed the authors to identify BamA (YaeT) as the outer membrane receptor for CDI and AcrB as a potential downstream target. PMID: 18761695
- 1H, 13C and 15N chemical shift assignments and secondary structure of the YaeT POTRA domain; a domain found in the Omp85 family of proteins which is critical for insertion and folding of outer membrane proteins in Gram-negative bacteria PMID: 19636842
顯示更多
收起更多
-
亞細胞定位:Cell outer membrane.
-
蛋白家族:BamA family
-
數據庫鏈接:
KEGG: ecj:JW0172
STRING: 316385.ECDH10B_0157
Most popular with customers
-
Recombinant Macaca mulatta Semaphorin-4D isoform 1 (SEMA4D), partial (Active)
Express system: Mammalian cell
Species: Macaca mulatta (Rhesus macaque)
-
Recombinant Rat Microtubule-associated protein tau (Mapt) (Active)
Express system: Mammalian cell
Species: Rattus norvegicus (Rat)
-
Recombinant Human Somatostatin receptor type 2 (SSTR2)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
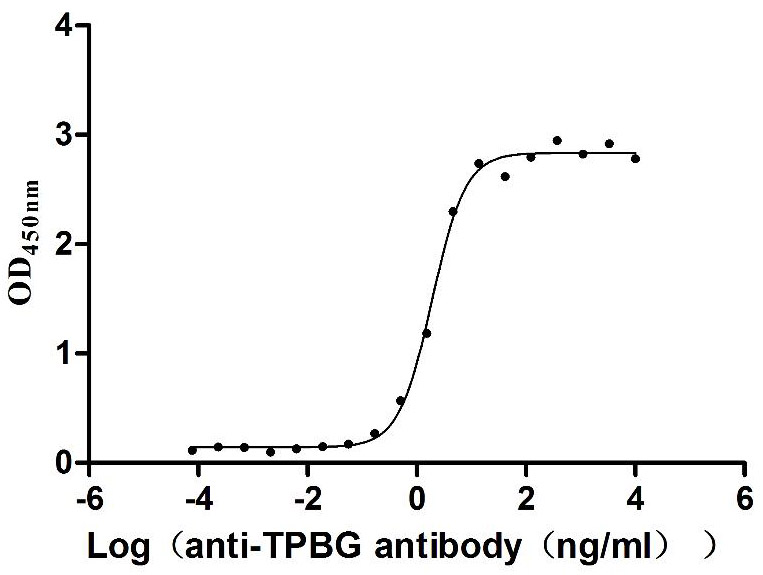
Recombinant Macaca fascicularis Trophoblast glycoprotein (TPBG), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
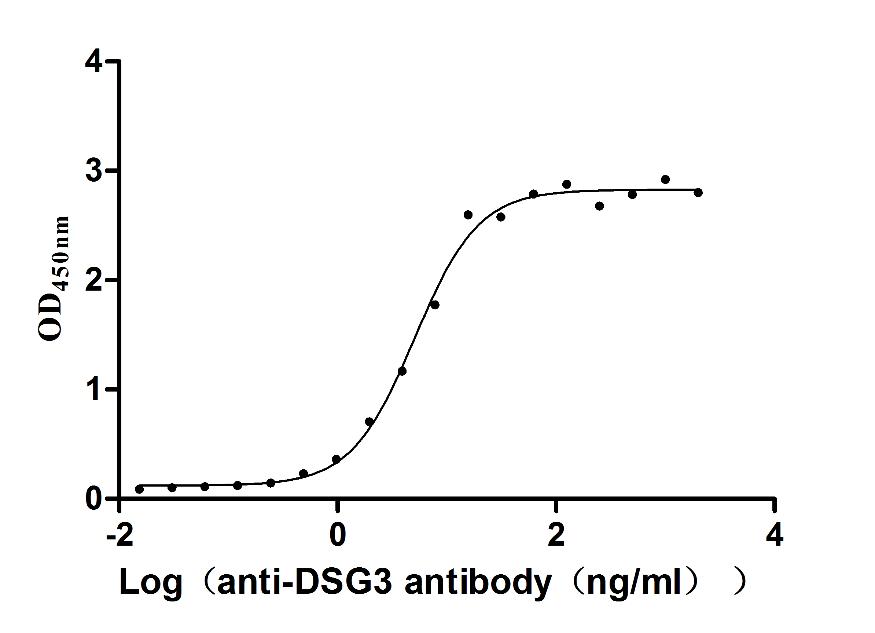
Recombinant Human Desmoglein-3 (DSG3), partial (Active)
Express system: Baculovirus
Species: Homo sapiens (Human)
-
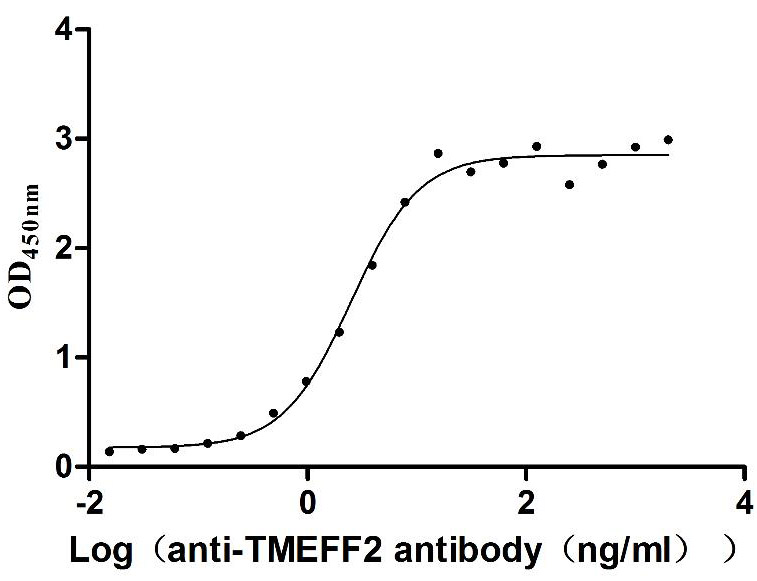
Recombinant Human Tomoregulin-2 (TMEFF2), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human C-C chemokine receptor type 8 (CCR8)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Macaca fascicularis Zinc transporter ZIP6 isoform X1(SLC39A6),partial (Active)
Express system: Baculovirus
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)