Recombinant Bovine Heat shock cognate 71 kDa protein (HSPA8), partial
-
中文名稱:牛HSPA8重組蛋白
-
貨號(hào):CSB-YP010829BO
-
規(guī)格:
-
來(lái)源:Yeast
-
其他:
-
中文名稱:牛HSPA8重組蛋白
-
貨號(hào):CSB-EP010829BO
-
規(guī)格:
-
來(lái)源:E.coli
-
其他:
-
中文名稱:牛HSPA8重組蛋白
-
貨號(hào):CSB-EP010829BO-B
-
規(guī)格:
-
來(lái)源:E.coli
-
共軛:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名稱:牛HSPA8重組蛋白
-
貨號(hào):CSB-BP010829BO
-
規(guī)格:
-
來(lái)源:Baculovirus
-
其他:
-
中文名稱:牛HSPA8重組蛋白
-
貨號(hào):CSB-MP010829BO
-
規(guī)格:
-
來(lái)源:Mammalian cell
-
其他:
產(chǎn)品詳情
-
純度:>85% (SDS-PAGE)
-
基因名:
-
Uniprot No.:
-
別名:HSPA8; HSC70; Heat shock cognate 71 kDa protein; Heat shock 70 kDa protein 8
-
種屬:Bos taurus (Bovine)
-
蛋白長(zhǎng)度:Partial
-
蛋白標(biāo)簽:Tag?type?will?be?determined?during?the?manufacturing?process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
產(chǎn)品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
復(fù)溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
儲(chǔ)存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質(zhì)期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事項(xiàng):Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
靶點(diǎn)詳情
-
功能:Molecular chaperone implicated in a wide variety of cellular processes, including protection of the proteome from stress, folding and transport of newly synthesized polypeptides, activation of proteolysis of misfolded proteins and the formation and dissociation of protein complexes. Plays a pivotal role in the protein quality control system, ensuring the correct folding of proteins, the re-folding of misfolded proteins and controlling the targeting of proteins for subsequent degradation. This is achieved through cycles of ATP binding, ATP hydrolysis and ADP release, mediated by co-chaperones. The co-chaperones have been shown to not only regulate different steps of the ATPase cycle of HSP70, but they also have an individual specificity such that one co-chaperone may promote folding of a substrate while another may promote degradation. The affinity of HSP70 for polypeptides is regulated by its nucleotide bound state. In the ATP-bound form, it has a low affinity for substrate proteins. However, upon hydrolysis of the ATP to ADP, it undergoes a conformational change that increases its affinity for substrate proteins. HSP70 goes through repeated cycles of ATP hydrolysis and nucleotide exchange, which permits cycles of substrate binding and release. The HSP70-associated co-chaperones are of three types: J-domain co-chaperones HSP40s (stimulate ATPase hydrolysis by HSP70), the nucleotide exchange factors (NEF) such as BAG1/2/3 (facilitate conversion of HSP70 from the ADP-bound to the ATP-bound state thereby promoting substrate release), and the TPR domain chaperones such as HOPX and STUB1. Plays a critical role in mitochondrial import, delivers preproteins to the mitochondrial import receptor TOMM70. Acts as a repressor of transcriptional activation. Inhibits the transcriptional coactivator activity of CITED1 on Smad-mediated transcription. Component of the PRP19-CDC5L complex that forms an integral part of the spliceosome and is required for activating pre-mRNA splicing. May have a scaffolding role in the spliceosome assembly as it contacts all other components of the core complex. Binds bacterial lipopolysaccharide (LPS) and mediates LPS-induced inflammatory response, including TNF secretion by monocytes. Participates in the ER-associated degradation (ERAD) quality control pathway in conjunction with J domain-containing co-chaperones and the E3 ligase STUB1. Interacts with VGF-derived peptide TLQP-21.
-
基因功能參考文獻(xiàn):
- Single-particle fluorescence imaging tracks the dynamics of Hsc70 and its clathrin substrate in real time. PMID: 21278753
- The interaction of the molecular chaperone Hsc70 (HSPA8) with recombinant PrP was investigated. PMID: 20434583
- Structure of clathrin coat with bound Hsc70 and auxilin. PMID: 20033059
- specific association between HSP73 and gentamicin may reduce the chaperone activity of HSP73 in vitro and/or in vivo PMID: 14966137
- report role of HSC70 in the regulation of NMT PMID: 15156568
- studied the process of disassembly by using cryo-electron microscopy to identify the initial binding site of Hsc70 on clathrin-C58J baskets at pH 6, under which conditions disassembly does not proceed further. Hsc70 interactions involve two sites PMID: 15596443
- Coats assembled from recombinant clathrin are good substrates for ATP- and auxilin-dependent, Hsc70-catalyzed uncoating. PMID: 17978091
- The structure of an Hsp110:Hsc70 nucleotide exchange complex, is reported. PMID: 18550409
- analysis of the formation of a stable complex between chaperonin-containing TCP-1 (CCT) and Hsc70 PMID: 18660820
- Elevated expression of bovine heat shock cognate (hsc)70 protein increases diabetes and inflammation following islet beta cell damage in a transgenic mouse model. PMID: 19812207
顯示更多
收起更多
-
亞細(xì)胞定位:Cytoplasm. Melanosome. Nucleus, nucleolus. Cell membrane.
-
蛋白家族:Heat shock protein 70 family
-
組織特異性:Ubiquitous.
-
數(shù)據(jù)庫(kù)鏈接:
Most popular with customers
-
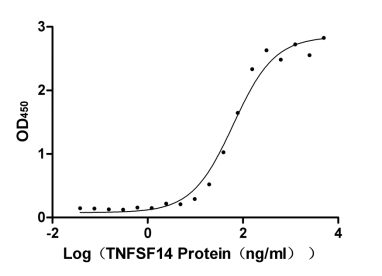
Recombinant Human Tumor necrosis factor receptor superfamily member 14 (TNFRSF14), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
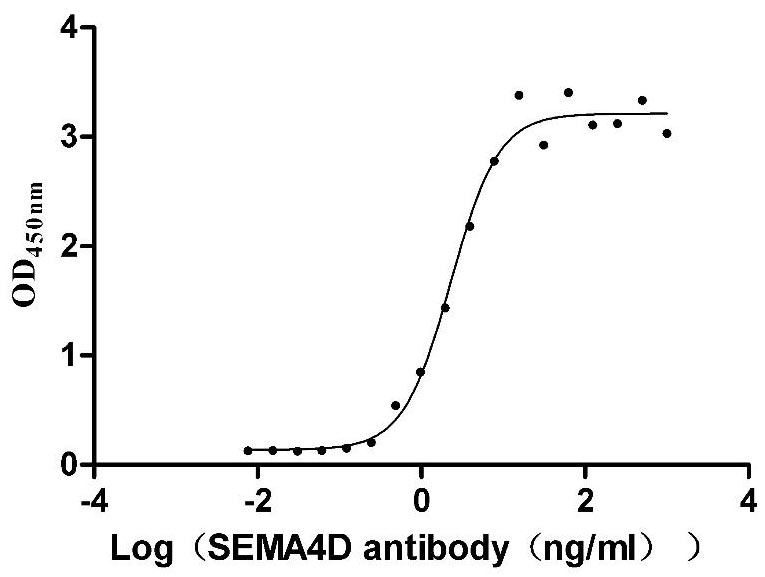
Recombinant Human Semaphorin-4D (SEMA4D), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
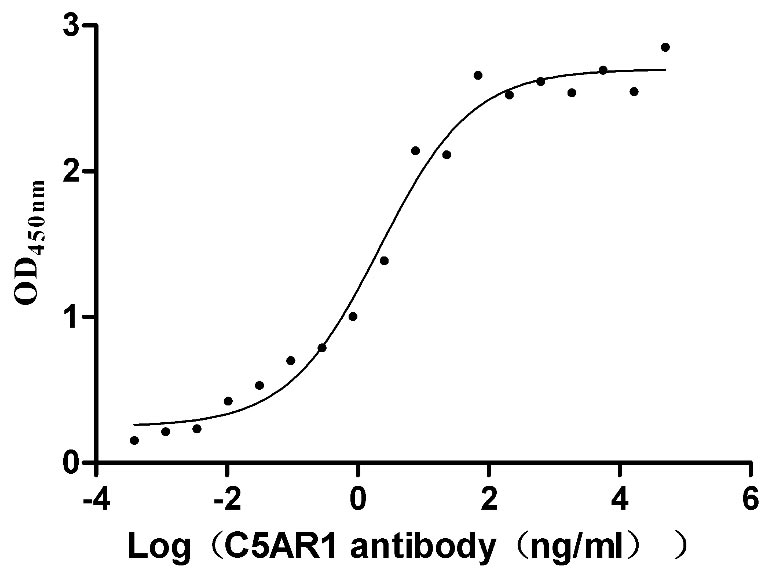
Recombinant Human C5a anaphylatoxin chemotactic receptor 1 (C5AR1)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Macaca mulatta Semaphorin-4D isoform 1 (SEMA4D), partial (Active)
Express system: Mammalian cell
Species: Macaca mulatta (Rhesus macaque)
-
Recombinant Human Alkaline phosphatase, germ cell type (ALPG) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
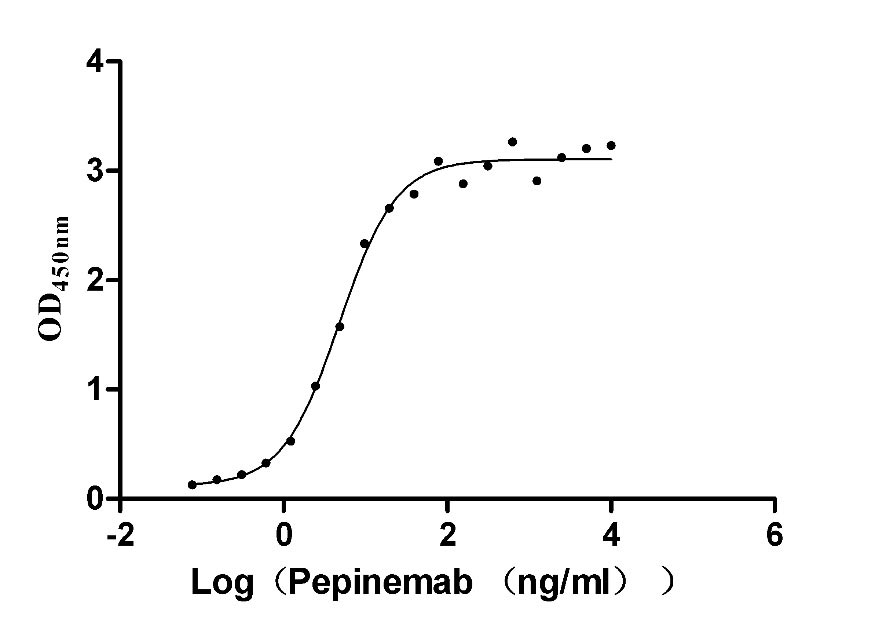
Recombinant Human B- and T-lymphocyte attenuator(BTLA), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Mucin-13(MUC13),partial (Active)
Express system: yeast
Species: Homo sapiens (Human)