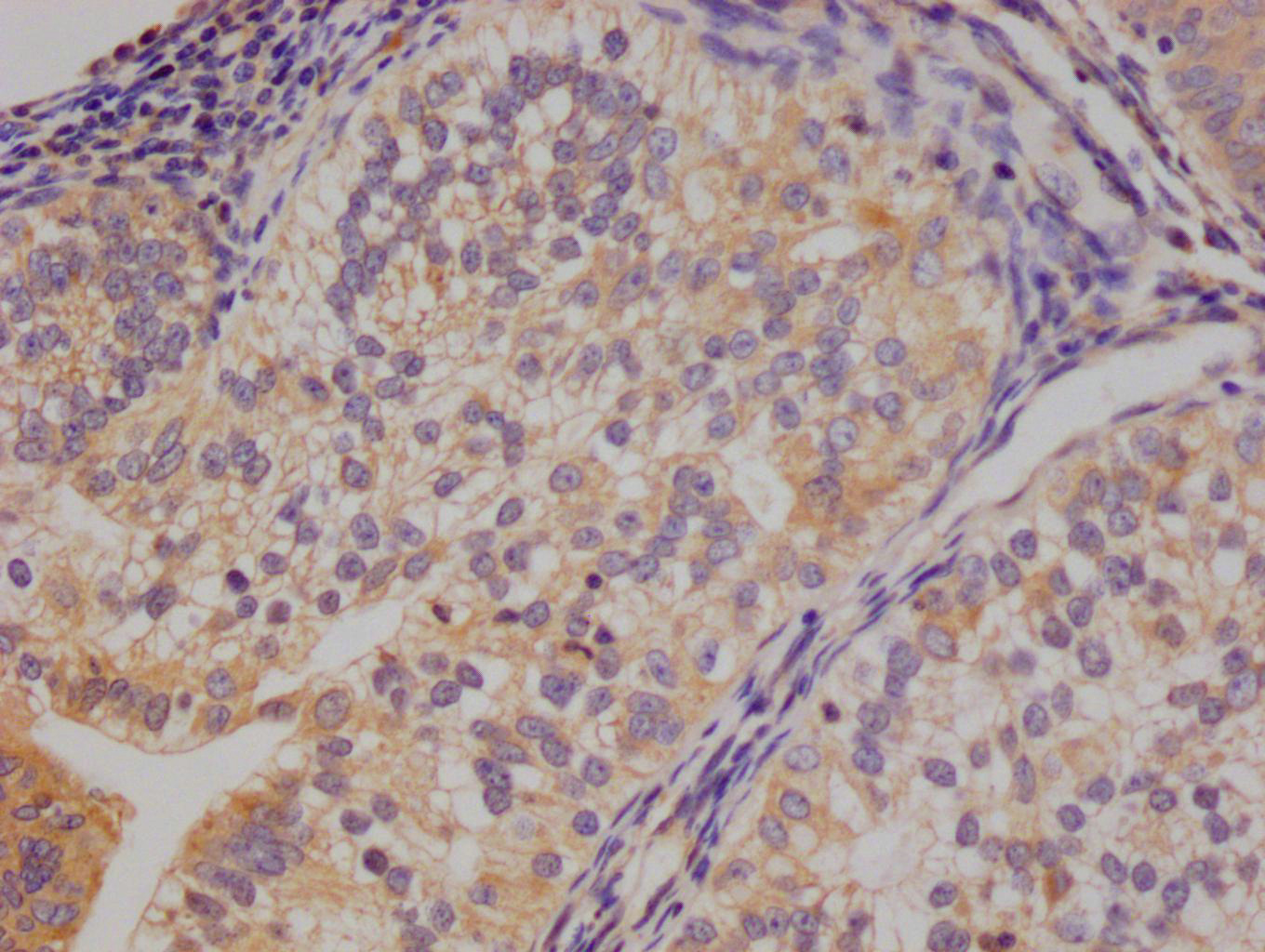
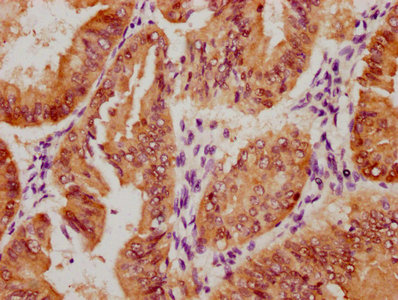
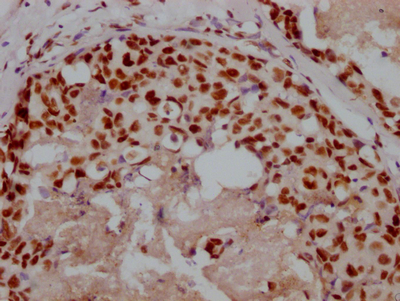
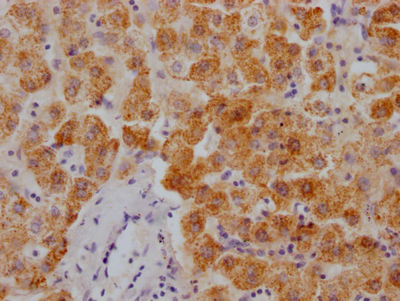
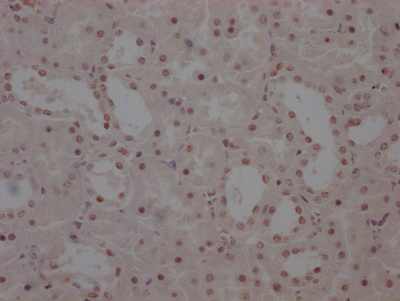
Tnni3 Antibody, Biotin conjugated
-
中文名稱:Tnni3兔多克隆抗體, Biotin偶聯
-
貨號:CSB-PA341785LD01MO
-
規格:¥880
-
其他:
產品詳情
-
產品名稱:Rabbit anti-Mus musculus (Mouse) Tnni3 Polyclonal antibody
-
Uniprot No.:
-
基因名:
-
別名:Tnni3 antibody; Troponin I antibody; cardiac muscle antibody; Cardiac troponin I antibody
-
宿主:Rabbit
-
反應種屬:Mouse
-
免疫原:Recombinant Mouse Troponin I, cardiac muscle protein (2-211AA)
-
免疫原種屬:Mus musculus (Mouse)
-
標記方式:Biotin
-
克隆類型:Polyclonal
-
抗體亞型:IgG
-
純化方式:>95%, Protein G purified
-
濃度:It differs from different batches. Please contact us to confirm it.
-
保存緩沖液:Preservative: 0.03% Proclin 300
Constituents: 50% Glycerol, 0.01M PBS, pH 7.4 -
產品提供形式:Liquid
-
應用范圍:ELISA
-
Protocols:
-
儲存條件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
貨期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
-
用途:For Research Use Only. Not for use in diagnostic or therapeutic procedures.
相關產品
靶點詳情
-
功能:Troponin I is the inhibitory subunit of troponin, the thin filament regulatory complex which confers calcium-sensitivity to striated muscle actomyosin ATPase activity.
-
基因功能參考文獻:
- Pim-1 is a novel kinase that phosphorylates cTnI primarily at Ser23/24 and Ser150 in cardiomyocytes, which in turn may modulate myofilament function under a variety of physiological and pathophysiological conditions. PMID: 29544221
- Hyperphosphorylation of this serine199 in cTnI C terminus impacts heart function by depressing diastolic function at baseline and limiting systolic reserve under physiological stresses. Paradoxically, it preserves heart function after ischemia/reperfusion injury, potentially by decreasing proteolysis of cTnI. PMID: 28899987
- The contributions of cardiac myosin binding protein C and troponin I phosphorylation to beta-adrenergic enhancement of in vivo cardiac function PMID: 26635197
- The difference in myosin regulatory light chain phosphorylation between the ventricles of R21C(+/+) in cardiac troponin I mice likely contributes to observed differences in contractile force and the lower tension monitored in the LV of HCM mice PMID: 25961037
- troponin I phosphorylation specifically alters the Ca(2+) sensitivity of isometric tension and the time course of relaxation in cardiac muscle myofibrils PMID: 25418306
- Combined troponin I Ser-150 and Ser-23/24 phosphorylation sustains thin filament Ca(2+) sensitivity playing an adaptive role to preserve contraction during acidic ischemia. PMID: 24657721
- these results indicate that the inability to enhance myofilament relaxation through cTnI phosphorylation predisposes the heart to abnormal diastolic function, reduced accessibility of cardiac reserves, dysautonomia, and hypertrophy. PMID: 24973218
- Dominant negative TnI-TnT interface mutation decreases the binding affinity of cTnI for TnT, causes early ventricular remodeling, and blunts the beta-adrenergic response of cardiac myocytes. PMID: 24898585
- R193H and R205H mutation increase the binding affinity of Troponin I for Troponin T and Troponin C. PMID: 24326031
- Conclude that dilated cardiomyopathy-causing mutations in thin filament proteins abolish the relationship between myofilament Ca(2+) sensitivity and troponin I phosphorylation by PKA. PMID: 23539503
- The pattern of cTnI post-translational modification depends on sex and hypertrophic cardiomyopathy genotype. PMID: 23352598
- A new functional and pathological role of amino acid modifications in the N-terminal acidic domain of cardiac TnI has been found that is modified by phosphorylations at TnI(S23/S24). PMID: 22940544
- Data show that cardiac TnI gene transition and the alternatively spliced cardiac TnT isoform switching occur in postnatal pulmonary vein. PMID: 23176202
- Conclude that cTnI phosphorylation by AMPK may represent a novel mechanism of regulation of cardiac function. PMID: 22456184
- Generation and functional characterization of knock-in mice harboring the cardiac troponin I-R21C mutation associated with hypertrophic cardiomyopathy. PMID: 22086914
- Data suggest that AMPK emerges as a possibly important regulator of cardiac and skeletal contractility via phosphorylation of a preferred site adjacent to the inhibitory loop of the thin filament protein TnI. PMID: 21416543
- Loss of troponin I leads to myofibril hypersensitivity to Ca(2+) causing impaired relaxation in restrictive cardiomyopathy. PMID: 20580639
- the functional effect of cTnI mutation and its potential value in compensating for the cTnT abnormality PMID: 20551314
- Ca(2+) binding to thin filaments reconstituted with either cTnI(wild-type) or pseudo-phosphorylated cTnI(S23D/S24D), cTnI(T144E), and cTnI(S23D/S24D/T144E) was determined. PMID: 20164197
- Studies indicate that that immunization of genetically susceptible mice with troponin I but not troponin T induced a robust autoimmune response leading to marked inflammation and fibrosis in the myocardium. PMID: 19446498
- calcium induces an extended conformation of the inhibitory region of troponin I in cardiac muscle troponin PMID: 11724531
- regulation of myocyte twitch kinetics by beta-stimulation and by endothelin-1 was altered in myocytes containing mutant cTnI PMID: 11934831
- PKC-mediated phosphorylation of Ser(43) and Ser(45) of cTnI plays an important role in regulating force development in the intact myocardium PMID: 12003851
- Troponin I serines 43/45 and regulation of cardiac myofilament function. PMID: 12181153
- demonstration of novel site specificity of effects of protein kinase C phosphorylation on function and emphasize the complexity of modulation of the actin-myosin interaction by specific changes in the thin filament PMID: 12551921
- the relationship between sarcomere length and myofilament lattice spacing in troponin I transgenic mice was markedly shifted downward to an overall decreased myofilament lattice spacing following protein kinase a treatment. PMID: 12562915
- A primary role of PKC phosphorylation of cTnI may be to reduce the requirements of the contractile apparatus for both Ca2+ and ATP, thereby promoting efficient ATP utilisation during contraction. PMID: 12923217
- autoantibodies to cTnI induce heart dysfunction and dilatation by chronic stimulation of Ca2+ influx in cardiomyocytes PMID: 14595408
- PKC-dependent phosphorylation of TnI has important role in the modulation of cardiac function under basal as well as augmented states PMID: 14726296
- cTnI has a pivotal role in the positive inotropic response of the murine heart to beta-adrenergic stimulation. PMID: 14966306
- protein kinase C phosphorylation of cardiac troponin I plays a dominant role in depressing contractility PMID: 15507454
- In conclusion, these data (alpha-chloralose-urethane) demonstrate that alpha-adrenergic-mediated force reduction is mediated through troponin I protein kinase C phosphorylation PMID: 15579573
- removal of the N-terminal extension of cTnI enhances cardiac function by increasing the rate of myocardial relaxation and lowering left ventricular end diastolic pressure to facilitate ventricular filling PMID: 15611140
- phosphorylation is driven by p90RSK PMID: 15840586
- The Ca2+ binding properties of various assemblies of the regulatory components that contain one of the cardiomyopathy-related mutant cTnI. PMID: 16531415
- Abnormal TnI phosphorylation observed in cardiac failure may explain exacerbated relaxation delay in response to increased afterload and contribute to blunted chronotropic reserve. PMID: 16936010
- The cTnI-G203S mutation disrupts interactions with partner proteins, and results in intracellular Ca2+ dysregulation early in life, suggesting a pathogenic role in development of familial hypertrophic cardiomyopathy. PMID: 16950368
- TnI deficiency impairs left ventricular relaxation, which leads to diastolic heart failure. PMID: 17526646
- cTnI-Cre mice have delayed onset of Cre activity during early heart development PMID: 17540338
- key role of cTnI in myocyte relaxation PMID: 17615373
- The primary effect of protein kinase A phosphorylation of cardiac troponin I is reduced Ca(2+) sensitivity of force, whereas phosphorylation of cardiac myosin-binding protein C accelerates the kinetics of force development. PMID: 17641226
- Changes in Ca(2+) affinity also support the idea that the equilibrium between states of actin-tropomyosin-troponin was shifted to the inactive state by mutations that mimic troponin I phosphorylation. PMID: 17872964
- Thr144 in cardiac TnI modulates cardiac myofilament length-dependent activation. PMID: 17975107
- Lys184 deletion in troponin I impairs relaxation kinetics and induces hypercontractility in murine cardiac myofibrils. PMID: 18096573
- Simultaneous defects in MHC7 & TnI accelerate onset & progression of familial hypertrophic cardiomyopathy. Compared with single-mutant models, double-mutant mice develop severe disease & premature death, progressing directly to a dilated phenotype. PMID: 18362229
- Impaired relaxation is the main manifestation in transgenic mice expressing a restrictive cardiomyopathy mutation, R193H, in cardiac TnI. PMID: 18408133
- Removal of the N-terminal extension of cardiac troponin I as a functional compensation for impaired myocardial beta-adrenergic signaling PMID: 18815135
- Transfer of troponin I-specific T cells can induce inflammation and fibrosis in wild-type mice, leading to deterioration of contractile function. Two sequence motifs of cTnI that induce inflammation and fibrosis in myocardium are characterized. PMID: 18955666
- These results indicate that YY1 is a novel regulator of fetal TnI transcription in the heart. PMID: 19013134
- the nNOS-PMCA4b complex regulates contractility via cAMP and phosphorylation of both PLB and cTnI. PMID: 19278978
顯示更多
收起更多
-
蛋白家族:Troponin I family
-
數據庫鏈接:
Most popular with customers
-
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-
-