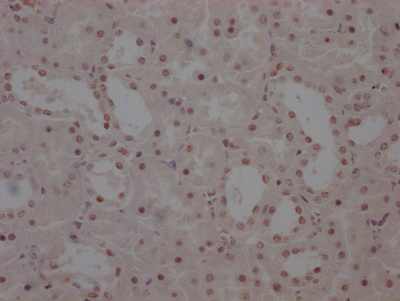
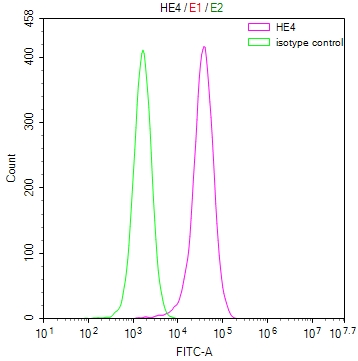
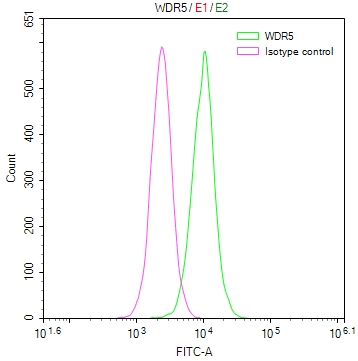
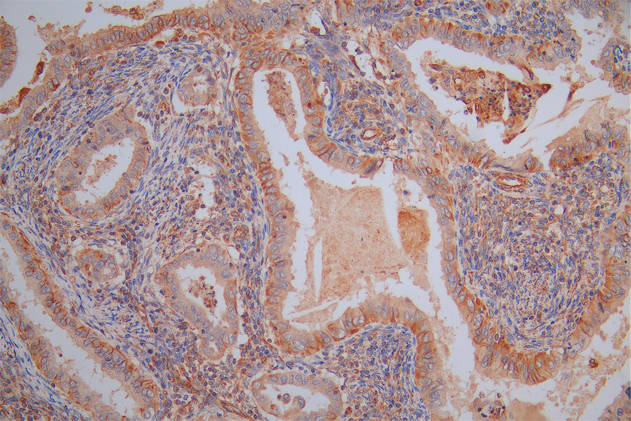
Phospho-RFWD2 (S387) Antibody
-
中文名稱:磷酸化-RFWD2 (S387)兔多克隆抗體
-
貨號:CSB-PA050025
-
規格:¥1090
-
其他:
產品詳情
-
Uniprot No.:
-
基因名:
-
別名:COP1; RFWD2; RNF200; E3 ubiquitin-protein ligase COP1; Constitutive photomorphogenesis protein 1 homolog; hCOP1; RING finger and WD repeat domain protein 2; RING finger protein 200; RING-type E3 ubiquitin transferase RFWD2
-
宿主:Rabbit
-
反應種屬:Human,Mouse
-
免疫原:Synthesized peptide derived from Human COP1 around the phosphorylation site of S387.
-
免疫原種屬:Homo sapiens (Human)
-
標記方式:Non-conjugated
-
抗體亞型:IgG
-
純化方式:The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
-
濃度:It differs from different batches. Please contact us to confirm it.
-
保存緩沖液:Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
-
產品提供形式:Liquid
-
應用范圍:WB, ELISA
-
推薦稀釋比:
Application Recommended Dilution WB 1:500-1:2000 ELISA 1:5000 -
Protocols:
-
儲存條件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
貨期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
-
用途:For Research Use Only. Not for use in diagnostic or therapeutic procedures.
相關產品
靶點詳情
-
功能:E3 ubiquitin-protein ligase that mediates ubiquitination and subsequent proteasomal degradation of target proteins. E3 ubiquitin ligases accept ubiquitin from an E2 ubiquitin-conjugating enzyme in the form of a thioester and then directly transfers the ubiquitin to targeted substrates. Involved in JUN ubiquitination and degradation. Directly involved in p53 (TP53) ubiquitination and degradation, thereby abolishing p53-dependent transcription and apoptosis. Ubiquitinates p53 independently of MDM2 or RCHY1. Probably mediates E3 ubiquitin ligase activity by functioning as the essential RING domain subunit of larger E3 complexes. In contrast, it does not constitute the catalytic RING subunit in the DCX DET1-COP1 complex that negatively regulates JUN, the ubiquitin ligase activity being mediated by RBX1. Involved in 14-3-3 protein sigma/SFN ubiquitination and proteasomal degradation, leading to AKT activation and promotion of cell survival. Ubiquitinates MTA1 leading to its proteasomal degradation. Upon binding to TRIB1, ubiquitinates CEBPA, which lacks a canonical COP1-binding motif.
-
基因功能參考文獻:
- COP1 overexpression inhibits p53 expression induced by fludarabine and promotes ubiquitin-mediated p53 degradation in chronic lymphocytic leukemia cells. PMID: 30423551
- miR-103 blocked PI3K/AKT signal pathway by regulation of COP1. These data indicated that miR-103 was up-regulated in drug resistant cells and it may regulate ADR-resistance by regulation of COP1 in AML cells. PMID: 29058777
- COP1 regulates human breast cancer cell proliferation and apoptosis in a p53-dependent manner.The COP1-mediated degradation of p53 regulates cancer cell growth and apoptosis. PMID: 29516369
- that COP1 may play a role in promoting glioma cell proliferation by interacting with and downregulating tumor suppressor p53 rather than oncogenic protein c-JUN PMID: 27534417
- COP1 forms complex with p53 protein and plays a role in p53 down-regulation. PMID: 29379285
- Authors demonstrate that mtp53 prevents the COP1/DET1 complex from ubiquitinating ETS2 and thereby marking it for destruction. Authors show that mtp53 destabilizes DET1 and also disrupts the DET1/ETS2 complex thereby preventing ETS2 degradation. PMID: 26871468
- STK40 binds the COP1 WD40 domain using a VPD/E motif in its C-terminal tail. PMID: 28089446
- In conclusion, miR-214 functions as a tumor suppressor by regulating the RFWD2-p53 cascade, thus delivery of miR-214 analogs could be a potential adjunct therapy in breast cancer harboring wild type p53. PMID: 27422604
- the reduced expression of COP1 and the upregulated expression of ETV1 in RCC tissue samples, which was associated with a high tumor-node-metastasis stage of RCC. Furthermore, the overexpression of COP1 in the RCC ACHN cells inhibited the migration and invasion of ACHN cells, and downregulated ETV1 and MMP7 expression levels. PMID: 27278120
- COP1 expression was an independent predictor of overall survival. PMID: 26753957
- protein level changes lead to increased sensitivity toward cisplatin treatment, implicating that huCOP1 plays a positive role in maintaining genome integrity in human keratinocytes. PMID: 27995412
- the present study revealed that COP1 plays an important role in CLL cell proliferation and tumorigenicity, and may be a useful indicator of the chronic lymphocytic leukemia processes. PMID: 26717976
- COP1 directly interacts with p27 through a VP motif on p27 and functions as an E3 ligase of p27 to accelerate the ubiquitin-mediated degradation of p27. COP1-p27 axis deregulation is involved in tumorigenesis. PMID: 26254224
- COP1 overexpression leads to the cytoplasmic distribution of p27, thereby accelerating p27 degradation. PMID: 25945542
- COP1 negatively regulates ETV1 in patients with triple-negative breast cancer. PMID: 25884720
- changes in the expression of fast-responding early genes is modulated by huCOP1 in keratinocytes upon UVB irradiation PMID: 25169772
- Phosphorylation of the ETS1 and ETS2 transcriptional oncoproteins at specific serine or threonine residues creates binding sites for the COP1 tumor suppressor protein. PMID: 25117710
- while the role of COP1 in malignancies is controversial, our current data support that COP1 acts as a tumor suppressor in gastric cancer. PMID: 23933908
- co-expressing COP1 and active GSK3beta blocked in vitro cell growth/migration and in vivo metastasis of invasive breast cancer cells. PMID: 24027432
- COP1 physically interacted with PTP1B and suppressed PTP1B phosphatase activity as well as the association of PTP1B with IRbeta. PMID: 23439647
- Modulation of fatty acid synthase degradation by concerted action of p38 MAP kinase, E3 ligase COP1, and SH2-tyrosine phosphatase Shp2. PMID: 23269672
- High level of COP1 expression is associated with poor prognosis in primary gastric cancer. PMID: 23091414
- Data define the subcellular localization and regulation of COP1 after DNA damage and provide a mechanistic explanation for the notion that 14-3-3sigma's impact on the inhibition of p53 E3 ligases is an important step for p53 stabilization after DNA damage. PMID: 20843328
- data suggest that the CSN6-COP1 axis is involved in 14-3-3sigma degradation, and that deregulation of this axis will promote cell growth and tumorigenicity PMID: 21625211
- the ubiquitin ligase COP1 (also known as RFWD2) is a tumour suppressor that negatively regulates ETV1, ETV4 and ETV5; ETV1, which is mutated in prostate cancer more often, was degraded after being ubiquitinated by COP1 PMID: 21572435
- MDM2, MDMX, Pirh2 and COP1 might inhibit p53 activity synergistically in vivo. PMID: 20333547
- RFWD2 is associated with acute lung injury in mice PMID: 21297076
- Increased COP1 is associated with hepatocellular carcinoma. PMID: 20959491
- COP1 contributes to UVB-induced signaling in human keratinocytes PMID: 19741714
- DET1 promotes ubiquitination and degradation of c-Jun by assembling a multisubunit ubiquitin ligase containing DNA Damage Binding Protein-1 (DDB1), cullin 4A (CUL4A), Regulator of Cullins-1 (ROC1), and constitutively photomorphogenic-1 PMID: 14739464
- tumour-suppressor protein p53 is a COP1-interacting protein; COP1 is a critical negative regulator of p53 PMID: 15103385
- Results suggest that overexpression of COP1 contributes to the accelerated degradation of p53 protein in cancers and attenuates the tumor suppressor function of p53. PMID: 15492238
- in response to DNA damage, ATM phosphorylated COP1 on Ser(387) and stimulated a rapid autodegradation mechanism; ionizing radiation triggered an ATM-dependent movement of COP1 from the nucleus to the cytoplasm PMID: 16931761
- dynamic changes of the COP1/COP1D ratio provide an additional level of regulation of the half-life of the substrates of this E3 ligase under homeostatic or pathological conditions PMID: 17968316
- COP1 binds FoxO1, enhances its ubiquitination, and promotes its degradation via the ubiquitin-proteasome pathway. PMID: 18815134
- Disruption of the COP1-mediated proteolysis by ionizing radiation leads to MTA1 stabilization. PMID: 19805145
- The ubiquitin ligase COP1 is a critical negative regulator of p53 and transcriptionally inducible by p53. PMID: 15103385
顯示更多
收起更多
-
亞細胞定位:Nucleus speckle. Cytoplasm. Note=In the nucleus, it forms nuclear speckles.
-
蛋白家族:COP1 family
-
組織特異性:Ubiquitously expressed at low level. Expressed at higher level in testis, placenta, skeletal muscle and heart.
-
數據庫鏈接:
Most popular with customers
-
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-
-