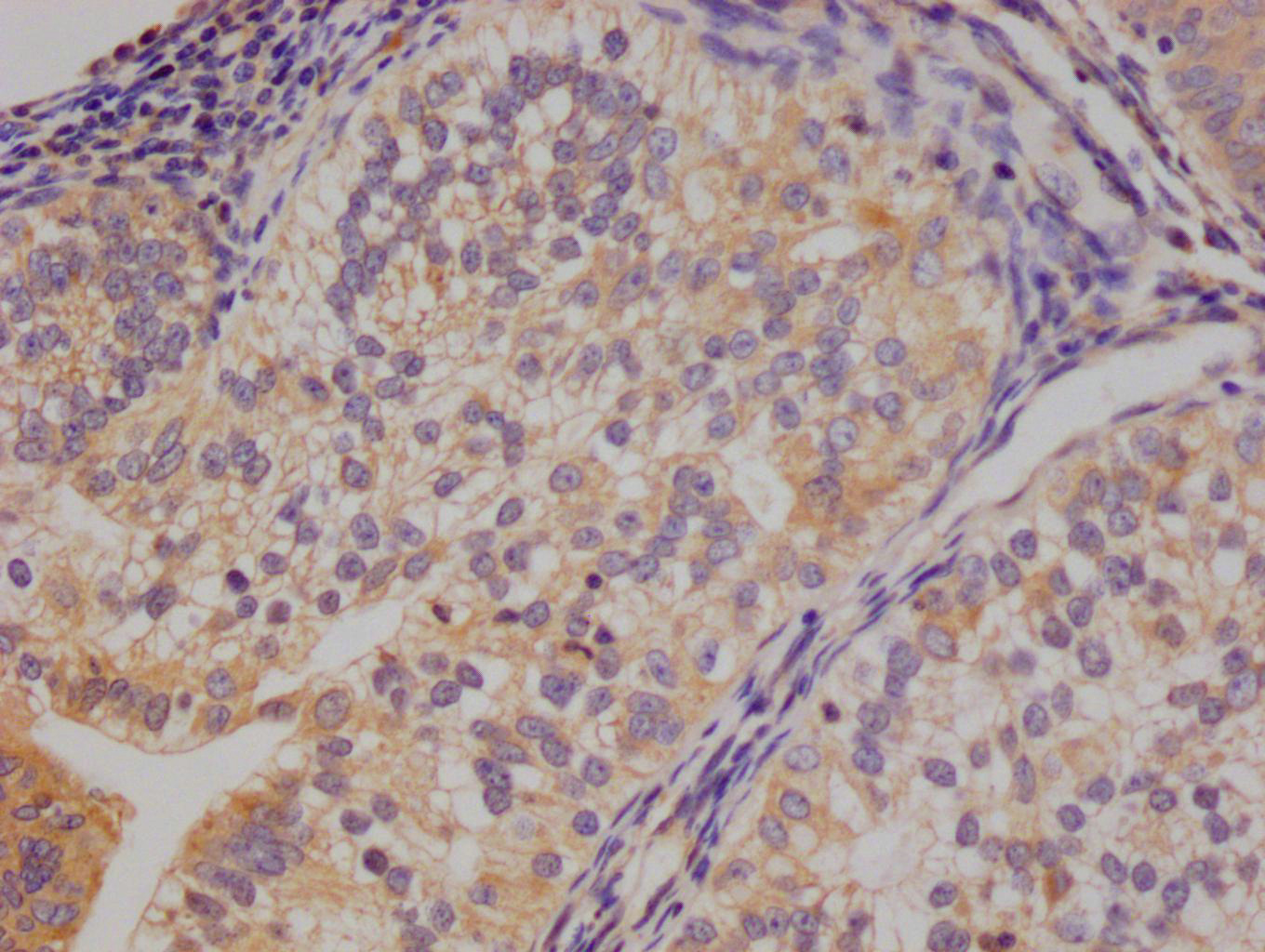
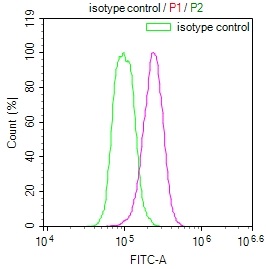
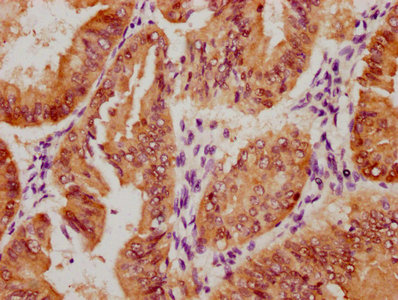
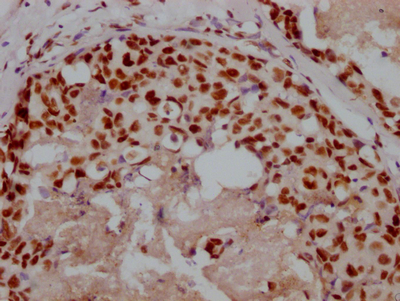
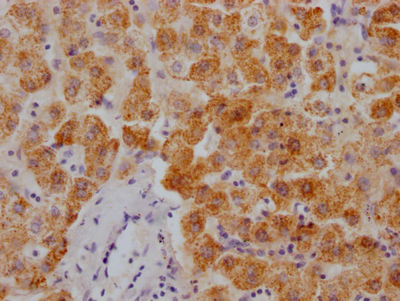
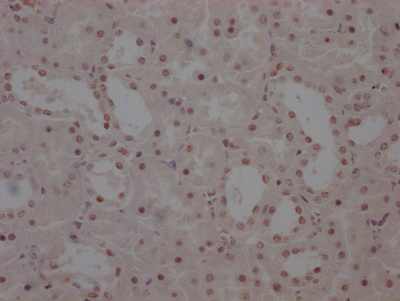
HSPB8 Antibody
-
中文名稱:HSPB8兔多克隆抗體
-
貨號:CSB-PA010839GA01HU
-
規(guī)格:¥3,900
-
其他:
產(chǎn)品詳情
-
Uniprot No.:
-
基因名:HSPB8
-
別名:Alpha crystallin C chain antibody; Alpha-crystallin C chain antibody; Charcot Marie Tooth disease axonal type 2L antibody; Charcot Marie Tooth disease spinal antibody; CMT2L antibody; CRYAC antibody; DHMN 2 antibody; DHMN2 antibody; E2 induced gene 1 protein antibody; E2-induced gene 1 protein antibody; E2IG1 antibody; H11 antibody; Heat shock 22kDa protein 8 antibody; Heat shock 27kDa protein 8 antibody; Heat shock protein 22 antibody; Heat shock protein beta 8 antibody; Heat shock protein beta-8 antibody; Hereditary motor neuropathy distal antibody; HMN 2 antibody; HMN2 antibody; HMN2A antibody; HSB8 antibody; HSPB 8 antibody; HspB8 antibody; HSPB8_HUMAN antibody; OTTHUMP00000239768 antibody; Protein kinase H11 antibody; Small stress protein like protein HSP22 antibody; Small stress protein-like protein HSP22 antibody; Spinal muscular atrophy distal adult autosomal dominant antibody
-
宿主:Rabbit
-
反應(yīng)種屬:Human,Mouse,Rat
-
免疫原:Human HSPB8
-
免疫原種屬:Homo sapiens (Human)
-
抗體亞型:IgG
-
純化方式:Antigen Affinity purified
-
濃度:It differs from different batches. Please contact us to confirm it.
-
保存緩沖液:PBS with 0.02% Sodium Azide, 50% Glycerol, pH 7.3. -20°C, Avoid freeze / thaw cycles.
-
產(chǎn)品提供形式:Liquid
-
應(yīng)用范圍:ELISA,WB,IHC
-
Protocols:
-
儲存條件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
貨期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
-
用途:For Research Use Only. Not for use in diagnostic or therapeutic procedures.
相關(guān)產(chǎn)品
靶點詳情
-
功能:Displays temperature-dependent chaperone activity.
-
基因功能參考文獻(xiàn):
- results strongly suggest that HSP22 represses HCC progression, especially HCC cell migration, by the down-regulation of the PI3K/AKT signaling pathway. PMID: 28456666
- Data indicated that the HSPB8 expression level was strongly positively correlated with MAPK signaling pathway and CREB pathway and worse prognosis. The methylation level of its DNA was negatively associated with its expression and positively associated with overall survival. These results suggest that HSPB8 could promote the proliferation and inhibit the apoptosis of GC cells by activating ERKCREB signaling. PMID: 29693129
- HSPB2 competes with HSPB8 for binding to BAG3. In contrast, HSPB3 negatively regulates HSPB2 association with BAG3. PMID: 28181153
- Silencing of HSPB8 markedly decreased the mitotic levels of BAG3 in HeLa cells, supporting its crucial role in BAG3 mitotic functions. The results support a role for the HSPB8-BAG3 chaperone complex in quality control of actin-based structure dynamics that are put under high tension, notably during cell cytokinesis. PMID: 28275944
- HSPB8 is involved in regulating the cell cycle and cell migration in MCF-7 cells. PMID: 28060751
- suggest that HSPB8 may act as an intracellular factor against hepatitis C virus replication and that DNAJC5B has the same function, with more relevant results for genotype 3 PMID: 29182667
- It has been demonstrated that HSPB8-BAG3-HSP70 ensures the functionality of stress granules and restores proteostasis by targeting defective ribosomal products for degradation. PMID: 27570075
- HSPB8 counteracts accumulation of aberrantly localized misfolded forms of TDP-43 and its 25 KDa fragment involved in most sporadic cases of Amyotrophic Lateral Sclerosis and of Fronto Lateral Temporal Dementia. PMID: 26961006
- expands the understanding of disease mechanisms, tissue involvement, and phenotypic outcome of HSPB8 mutations PMID: 26718575
- our findings suggest the existence of a so-far unrecognized quality control mechanism involving BAG3, HSPB8 and p62/SQSTM1 for accurate remodelling of actin-based mitotic structures that guide spindle orientation. PMID: 26496431
- This study demonstreated that expression of HSPB8 was restricted to GFAP+ astrocytes in patient with multiple sclerosis. PMID: 26694816
- Pangenomic profiling of velcade-sensitive and resistant cells showed that the small heat shock protein HSPB8 was overexpressed in multiple myeloma resistant cells. PMID: 25051369
- HSP22 acts as a positive regulator in TGF-alpha-induced migration of ovarian cancer cells, subsequently directing ovarian cancer toward progression. PMID: 25731856
- HSP22 plays an important role on gastric tumor aggressiveness and prognosis and may act as a promising target for prognostic prediction. PMID: 24804817
- Mutant HSPB8 causes protein aggregates and a reduced mitochondrial membrane potential in dermal fibroblasts from distal hereditary motor neuropathy patients. PMID: 22595202
- The expression of HspB8 inhibits the growth of genetically diverse melanoma cells that include caspase-1 activation outside of the realm of the inflammasome, mTORC1-dependent Beclin-1 upregulation and its cleavage by the activated caspase-1. PMID: 22898869
- findings show that during heat shock recovery NF-kappaB activates selective removal of misfolded or aggregated proteins by controlling expression of BAG3 and HSPB8 and by modulating the level of the BAG3-HspB8 complex PMID: 22302993
- we observed upregulation of HSPB8 and BAG3 selectively in astrocytes located within the degenerated areas of patients with protein aggregation diseases PMID: 21696420
- We studied the HSPB1 and HSPB8 mutation occurrence in patients with distal hereditary motor neuropathy and those with the axonal form of Charcot-Marie-Tooth disease type 2 PMID: 22176143
- present study demonstrates that HSPB8 is silenced by DNA methylation in hematopoietic malignant and normal cells PMID: 21914495
- The results of this syudy suggested that defects in HspB8-mediated autophagy are likely to contribute to dHMNII pathology and their detection in peripheral blood mononuclear cells could be a useful, accessible biomarker for the disease. PMID: 21985219
- HSPB8 may play an important role in the protection of cells under lethal heat shock treatment, and the K141N mutation can impair the protective effect. PMID: 21983727
- phosphorylation of HspB8 by ERK1 might be important for regulation of interaction of HspB8 with different target proteins PMID: 21526341
- Overexpression of HSPB1, as well as HSPB6, HSPB7 and HSPB8 independently protect against tachycardia remodeling by attenuation of the RhoA GTPase pathway at different levels. PMID: 21731611
- The complexes formed by Bag3 and HspB8 might have variable stoichiometry and can participate in different processes including clearing of the cell from improperly folded proteins. PMID: 21767525
- Drosophila HSP67Bc is the functional ortholog of human HSPB8 and Dm-HSP67Bc induces autophagy via the eIF2alpha pathway. PMID: 20858900
- HspB8 increases misfolded SOD1 clearance via autophagy. PMID: 20570967
- Data show that the interaction between HspB6 and Bag3 requires the same regions that are involved in the HspB8-Bag3 association. PMID: 19845507
- The results confirm predictions that Hsp22 belongs to the family of intrinsically disordered proteins with certain parts of its molecule retaining folded structure and undergoing reversible thermal unfolding. PMID: 19783089
- Forced H11 expression triggers apoptosis. PMID: 12832417
- In two pedigrees with distal hereditary motor neuropathy type II linked to chromosome 12q24.3, we identified the same mutation (K141N) in small heat-shock 22-kDa protein 8 (encoded by HSPB8; also called HSP22). PMID: 15122253
- Hsp22 is highly homologous to small heat shock proteins and effectively prevents aggregation of denatured protein both in vitro and in vivo. It is supposed that chaperone-like activity is of great importance for Hsp22 functioning PMID: 15541337
- Reduced chaperone activity of mutated HspB8 is associated with neuromuscular disorders PMID: 15879436
- The rate of HSP22 gene mutation in Chinese patients with Charcot-Marie-Tooth disease is as low as 0.87%(1/115). PMID: 16086267
- Our results suggest that a variety of oligomers composed of different proportions of different sHSPs may form in cell types expressing multiple sHSPs. PMID: 16225851
- HspB8 might play important role in regulating Abeta aggregation and, therefore, development of classic senile plaques in Alzheimer's disease and cerebral amyloid angiopathy in hereditary cerebral hemorrhage with amyloidosis of Dutch type. PMID: 16485107
- Small heat shock protein B8 (HSP22) is a novel TLR4 agonist abundantly expressed in synovial tissue from patients with rheumatoid arthritis. PMID: 16709864
- found aberrantly increased interactions of neuropathy-associated mutant HSP22 forms with themselves, with wild-type HSP22, and with the other sHSPs, alphaB-crystallin, and HSP27. PMID: 16935933
- Aberrant DNA methylation silences the novel heat shock protein H11 in melanoma PMID: 17033167
- HspB8 overload causes melanoma growth arrest and apoptosis through TAK1 activation PMID: 17173073
- Displays chaperone activity, autokinase activity, and trigger or block apoptosis activity. Decrease may contribute to development of some neurologic diseases and others. (Review) PMID: 17304582
- HSP22 seems to play an important role in the nervous system. PMID: 17722063
- Effect of mutations in the beta5-beta7 loop on the structure and properties of human small heat shock protein HSP22 PMID: 17922839
- These results suggested that the HspB8-Bag3 complex might stimulate the degradation of Htt43Q by macroautophagy. PMID: 18006506
- HSPB8 is a candidate CDK-independent cyclin D1 target that can mediate its effects PMID: 18006821
- HspB8 and Bag3: a new chaperone complex targeting misfolded proteins to macroautophagy. PMID: 18094623
- Hsp22 induction represents a new aspect of the estrogenic response with potential significance for the biology of estrogen receptor-positive breast cancer cells. PMID: 18229450
- Mutations in serine residues of HSP22 which are phosphorylated by cAMP-dependent protein kinase were accompanied by decrease of chaperone-like activity. PMID: 18298377
- Activation of the bone morphogenetic protein receptors Alk3 and Bmpr2 by H11kinase/Hsp22 promotes cardiac cell growth and survival. PMID: 19246680
- H11 kinase is a novel mediator of myocardial hypertrophy in vivo PMID: 12456486
顯示更多
收起更多
-
相關(guān)疾病:Neuronopathy, distal hereditary motor, 2A (HMN2A); Charcot-Marie-Tooth disease 2L (CMT2L)
-
亞細(xì)胞定位:Cytoplasm. Nucleus. Note=Translocates to nuclear foci during heat shock.
-
蛋白家族:Small heat shock protein (HSP20) family
-
組織特異性:Predominantly expressed in skeletal muscle and heart.
-
數(shù)據(jù)庫鏈接:
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-